Preventing protein unfolding
When the body loses its ability to fold proteins into the correct shapes, the result can be irreversible and tragic. The accumulation of unfolded or misfolded proteins in the brain causes many devastating neurodegenerative diseases, including Alzheimer's, Parkinson's and amyotrophic lateral sclerosis (ALS).
In order to maintain their functions, structural proteins and engineered, protein-based materials need to avoid unfolding even under large mechanical stresses. Scientists, therefore, are exploring ways to design proteins that can survive extreme mechanical insults.
Northwestern Engineering's Sinan Keten has theoretically demonstrated that small proteins can be reinforced with covalently bonded polymers against mechanical unfolding. His computational model illustrates strategies for using this polymer conjugation to prevent proteins from rapidly unfolding even when stretched or pulled apart.
"If you apply a stress to a protein, we know it will start to unfold," said Keten, assistant professor of mechanical, civil and environmental engineering. "Given that proteins are subject to mechanical forces in the body and in all applications, it will be useful to reinforce them in this way."
Elizabeth DeBenedictis, a PhD student in Keten's lab, and Elham Hamed, a former postdoctoral fellow in Keten's lab, are the paper's first authors. DeBenedictis also created the painting that was used for the journal's cover image.
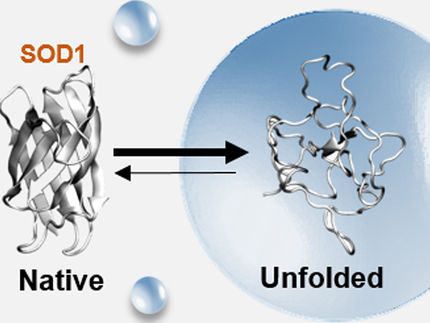
A protein's shape is related to its function. By coiling and folding into specific three-dimensional shapes, they are able to perform their different biological tasks. Proteins are held together by weak hydrogen bonds. When they unfold, these bonds break and are often replaced by hydrogen bonds with water.
"Once the water is in there, it's hard to reverse the process," Keten explained. "It's hard for the protein to refold."
Researchers have long known that attaching polymers to proteins can stabilize them thermally. But little is known from a mechanical perspective. Keten's team used a common protein structure, called an alpha helix, and a soft, nontoxic polymer called poly-ethylene-glycol to test the reinforcing strategy under mechanical forces. They found that, through hydrophobic and electrostatic interactions, the polymer can reside near the surface of the protein. This shields its backbone hydrogen bonds from being replaced by bonds with water molecules, enabling the protein to hold its specific shape much longer under constant stress.
"The protein can refold back to its original configuration more easily," he said. "When the polymer is close to the surface, you see refolding."
Not only could this finding inform medicine about how to treat or prevent protein unfolding diseases, but the method could be used to stabilize protein-based biomaterials, which is important giving vaccines longer shelf lives, improving drug delivery and creating stronger scaffolds for tissue engineering.
Next, Keten's team will create a design strategy for determining polymer and protein interfaces that work well together. The team also collaborates with experimental groups to explore applications that may benefit from Keten's computational models.
Original publication
Other news from the department science

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Hodgkin's_lymphoma
Intensive_insulinotherapy
Children's_Global_Assessment_Scale
Jean-Baptiste_Lamarck
Gemmules
Bromoethane
Intermittent_explosive_disorder
Male_lactation
Nematicide