Determining the structures of nanocrystalline pharmaceuticals by electron diffraction
Reliable information about the structure of pharmaceutical compounds is important for patient safety, for the development of related drugs and for patenting purposes. However, working out the structures of pharmaceuticals can be tough. The individual molecules can pack together in the solid in different ways to form different polymorphs, and pertinent properties such as stability, bioavailability or how fast they dissolve in the stomach can vary from one polymorph to another. Single crystals (as used in standard X-ray diffraction experiments) therefore might not be representative of the bulk sample, or indeed might not even be available.
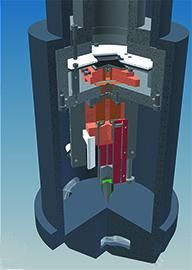
90° cut-off schematic of the camera as designed for the CM200 (Technische Universiteit Delft, The Netherlands).
van Genderen et al
Moreover, the compounds themselves can be damaged by the high energy of the X-radiation used. As electrons are less damaging than X-rays by several orders of magnitude, using electron diffraction should be an attractive alternative, particularly when only nanometre-sized crystals are available. Cooling the sample to liquid-nitrogen temperatures ('cryo-cooling') can also help to minimize radiation damage, but the compound might change structure on cooling, so the structure that is obtained is not actually that of the material as taken by the patient at room temperature.
A group of scientists from a number of European countries have tackled all aspects of these problems by using low-dose electron diffraction, rotating the sample so that individual nanocrystals are not in the electron beam long enough to be damaged and collecting the diffraction data using a new type of detector developed by CERN. This new detector combines a high dynamic range with a very high signal-to-noise ratio and sensitivity to single electrons. Radiation damage was reduced so much that cooling the sample was not found to be necessary, allowing the team to study the anticonvulsant drug carbamazepine and nicotinic acid (vitamin B3) at room temperature. The data they collected were high enough quality that they could solve the structures of the two compounds using direct methods and software developed for X-ray crystallography.
Based on their experience with these case studies, the authors are planning to improve the design of their experimental setup further, and will also be developing programs specifically designed for handling electron-diffraction data.
Original publication
E. van Genderen, M. T. B. Clabbers, P. P. Das, A. Stewart, I. Nederlof, K. C. Barentsen, Q. Portillo, N. S. Pannu, S. Nicolopoulos, T. Gruene and J. P. Abrahams; "Ab initio structure determination of nanocrystals of organic pharmaceutical compounds by electron diffraction at room temperature using a Timepix quantum area direct electron detector"; Acta Crystallographica Section A; 2016
Most read news
Original publication
E. van Genderen, M. T. B. Clabbers, P. P. Das, A. Stewart, I. Nederlof, K. C. Barentsen, Q. Portillo, N. S. Pannu, S. Nicolopoulos, T. Gruene and J. P. Abrahams; "Ab initio structure determination of nanocrystals of organic pharmaceutical compounds by electron diffraction at room temperature using a Timepix quantum area direct electron detector"; Acta Crystallographica Section A; 2016
Topics
Organizations
Other news from the department science

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.

















































