Demystifying mechanotransduction ion channels
Advertisement
As blood flows through our vessels, the cells that constitute these vessels responds to the shear stress of blood flow to ensure normal circulation. This process of converting a mechanical force into a biological function is known as "mechanotransduction."
Mechanotransduction
Bailong Xiao
But a bit of mystery has enshrouded the type of specialized mechanotransducers--force sensors--underlying the process and how they're able to sense a force and, subsequently, transduce to downstream biological functions.
During the Biophysical Society's 60th Annual Meeting, being held in Los Angeles, Calif., Feb. 27-March 2, 2016, Bailong Xiao, an associate professor at Tsinghua University's School of Pharmaceutical Sciences, in Beijing, China, will share a big discovery made while exploring how newly identified mechanotransducers function at the molecular level.
"Mechanosensitive channels represent a class of ion channels that respond to mechanical force stimulation and allow ions to enter or exit cells," explained Xiao. "They are suspected of serving as key mechanotransducers for mechanotransduction, but the molecular identities of mechanosensitive cation channels in mammals were unknown until the identification of the evolutionarily conserved Piezo family of proteins--including Piezo1 and Piezo2--by Dr. Ardem Patatpoutian's lab at the Scripps Research Institute in 2010."
Since then, it's been shown that Piezo proteins play critical roles in various mechanotransduction processes. "Piezo1, for example, plays a key role in sensing blood-flow-associated shear stress and, consequently, controlling vascular development and function," he said. "In humans, mutations of Piezo1 or Piezo2 genes have been linked to genetic diseases."
Piezo proteins are complex transmembrane proteins that don't possess notable sequence homology with any known class of ion channels. "These features make it difficult to study their structure-function relationship using traditional site-directed mutagenesis approaches," Xiao elaborated. "While working in Patatpoutian's lab as a postdoctoral fellow, we previously demonstrated that Piezo1 proteins form a novel class of ion channels." This work was published in the journal Nature in 2012, and Xiao was co-first author.
But fundamental questions remained unanswered, among them: How do these proteins three-dimensionally organize into mechanosensitive channels? How do they conduct ions and respond to force stimulation?
After setting up his own lab at Tsinghua University in Beijing, Xiao began tackling these questions.
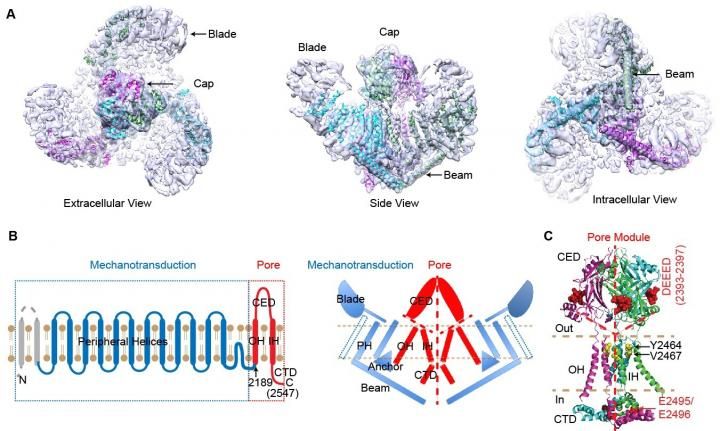
In a paper the group published in Nature in 2015, along with collaborators at the university, they reported resolving the cryo-electron microscopy (cryo-EM) structure of the full-length mouse Piezo1.
Now in a paper published online in Neuron on Feb. 25, 2016, the group reports "functionally identifying bona-fide ion-conducting pore and mechanotransduction components," Xiao said. "Our findings demonstrate that Piezo1 proteins consist of distinct and separate modules responsible for ion conduction, mechanical force sensing and transduction to coordinately fulfill their function as sophisticated mechanosensitive channels."
This is consistent with the structural organization of these functional modules into a unique three-bladed, propeller-shaped architecture of Piezo1, according to Xiao. "Our studies significantly advance our mechanistic understanding of how this evolutionarily conserved and physiologically important class of mechanosensitive channels respond to mechanical force and, consequently, conduct ions for biological functions," he added.
In terms of applications, his group's studies "help us understand the specialized force sensors such as Piezo1 ion channels that play critical roles in vascular development and blood cell function, which might enable us to design novel therapeutics in the future to treat diseases caused by abnormal functions of these mechanotransducers," Xiao said.
Next, Xiao and his group are planning more mechanistic studies to gain a better understanding of the mechanosensitive channels and to identify ways to manipulate their functions. "In the long run, we hope to develop novel therapeutics by targeting these mechanosensors," he said.