HSF1 – in case of emergency
Just as we humans do well to call the police or fire services in the event of an emergency, cells have helpers that are activated in a crisis. cellular stress activates heat-shock transcription factor 1 (HSF1), which then binds DNA and facilitates the production of the cellular helpers. Researchers from the Max Planck Institute of Biochemistry in Martinsried have managed to demonstrate how this process works. Using X-ray crystallography, the scientists have decoded the exact structure of HSF1 and are thus able to explain the protein’s operating mode.
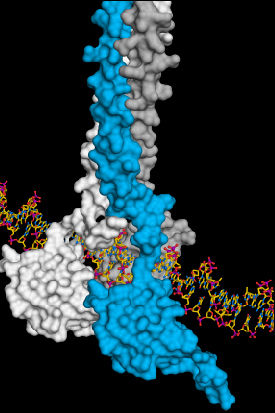
Three HSF1 molecules (white, blue, grey) associate to ensure stable interaction between HSF1 and DNA. This activates the production of cellular crisis helpers, the heat-shock proteins.
Tobias Neudegger, MPI of Biochemistry
When there is an accident or a house fire, we call the police or the fire services. A control room quickly coordinates emergency operations. The cells in our bodies also have helpers in a crisis; the heat-shock proteins. These are triggered in response to cellular stress, such as high temperature, UV radiation or cancer. Heat-shock proteins help other proteins maintain their functional structure and eliminate denatured proteins to counter the abnormal cellular situation.
In cells, the operator in the control room is HSF1, heat-shock transcription factor 1. It binds certain DNA sequences that encode the “assembly instructions” for the cellular helpers. When HSF1 is activated, the production of functional heat-shock proteins is triggered.
Andreas Bracher and his team in Prof. Hartl’s Department of Cellular Biochemistry at the Max Planck Institute of Biochemistry in Martinsried have demonstrated exactly how HSF1 binds DNA. “Using X-ray crystallography, we studied the exact molecular arrangement,” explains Tobias Neudegger, a member of Bracher’s team and first author of the study. Proteins consist of long strands of amino acids which adopt a certain three-dimensional structure in order to become functionally active.
“We were able to show how three identical HSF1 molecules associate in case of cellular stress. That is how a stable DNA-HSF1 interaction occurs. If HSF1 is not bound to DNA, each individual HSF1 molecule is stored in an inactive state in the cell,” Neudegger explains.
The increased production of heat-shock proteins could be advantageous for the treatment of diseases. “Now that we know the HSF1 structure, drugs can be developed to activate or deactivate HSF1 and thus stimulate or inhibit the production of cellular helpers,” says Bracher, describing potential future HSF1 research. Incorrectly folded proteins in the cells could be repaired or denatured proteins more easily eliminated. Incorrectly folded proteins are usually found in connection with Huntington’s disease, Alzheimer’s and Parkinson’s disease, as well as in cancer cells.
Original publication
Most read news
Original publication
T. Neudegger, J. Verghese, M. Hayer-Hartl, F. U. Hartl & A. Bracher; "Structure of human heat-shock transcription factor 1 in complex with DNA"; Nature Structural & Molecular Biology; 2016
Topics
Organizations
Other news from the department science

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.