Disruptions to embryonic reprogramming alter adult mouse behavior
Advertisement
Right after fertilization, embryos at the earliest stages of development tell their genes: "Forget what it was like in the sperm or egg where you came from."
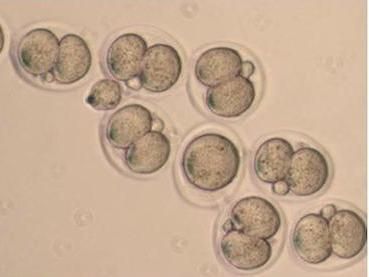
One and two cell mouse embryos
Jadiel Wasson
When the process of epigenetic reprogramming is defective in mouse development, the consequences in adulthood can include abnormal repetitive behaviors, scientists have shown.
"Our results demonstrate how defects in reprogramming may influence the development of altered behaviors, or even complex psychiatric disorders," says co-senior author David Katz, PhD, assistant professor of cell biology at Emory University School of Medicine.
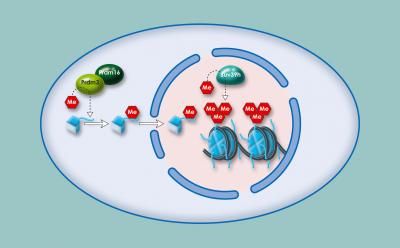
After fertilization, the enzyme KDM1A (lysine specific demethylase 1A) appears to act as an epigenetic eraser, wiping away information carried on histones, the spool-like proteins that package DNA. KDM1A removes histone methylation, a chemical modification that shapes the activity of nearby genes.
Katz and graduate student Jadiel Wasson created genetically engineered mice that have KDM1A missing from their mouse oocytes (or egg cells), but present later in development. They teamed up with Todd MacFarlan, PhD, previously at the Salk Institute and now at the National Institute of Child Health and Human Development, to examine several mouse strains with alterations in the KDM1A gene.
While these mice were created by genetic engineering techniques, Katz says they may simulate other disruptions of the reprogramming process after fertilization. Those disruptions might come from genetic changes or environmental influences on oocytes such as hormones or parental age, he says.
"These mice have functioning KDM1A later on in development, because they inherit a good copy of the gene from their fathers," Macfarlan says. "But it's not there at a critical stage -- what we call the maternal-to-zygote transition."
In the situation when egg cells are missing the enzyme completely, much of the pre-fertilization information present on histones persists after fertilization. That means thousands of egg- and sperm-specific genes are turned on that shouldn't be, and a similar number needed for embryonic development are turned off.
"Usually, that's lethal," Katz says. "The fertilized egg can't tolerate it at all, and is unable to divide beyond the two cell stage."
In contrast, in some of the mice Katz and Wasson studied, a bit of KDM1A enzyme remained in the oocytes. Although most of the resulting mice died in utero or right after birth, a few survived to adulthood. They looked like normal mice, but they behaved strangely.
Wasson says one aspect of their behavior became apparent when more than one was in a cage at the same time: they ground up much of their food and incorporated it into their bedding. The KDM1A-reduced mice also displayed excessive scratching and digging. When tested on marble burying, a task which has been used to gauge obsessive-compulsive behavior in other genetically modified mice, these mice were even more avid marble buriers than had been observed elsewhere.
What accounts for the strange behavior? Wasson and Katz are beginning to examine changes in the brains of the KDM1A-reduced mice. One possible contributor is changes they observed in imprinted genes. With imprinted genes, one copy is silenced depending on whether it comes from the mother or father; these patterns are disrupted in the KDM1A-reduced mice.
"The most likely scenario, and one that we are pursuing, is that the disruptions in gene expression ultimately influence the wiring of the brain," Katz says. "This could be via imprinting, but is just as likely to be via disruption of normal neuronal development."
To study KDM1A's role in brain development, Katz and Macfarlan are planning to create and examine mice that have a less drastic reduction of the KDM1A enzyme in oocytes, so that more survive to adulthood.