Investigational monoclonal antibody to treat Ebola is safe in adults
Advertisement
The investigational Ebola treatment mAb114 is safe, well-tolerated, and easy to administer, according to findings from an early-stage clinical trial. Eighteen healthy adults received the monoclonal antibody as part of a Phase 1 clinical trial that began in May 2018 at the National Institutes of Health (NIH) Clinical Center in Bethesda, Maryland. The National Institute of Allergy and infectious diseases (NIAID) Vaccine Research Center (VRC), part of NIH, developed the investigational treatment and conducted and sponsored the clinical trial.
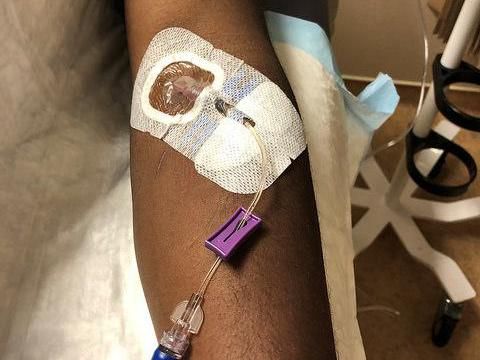
A healthy volunteer receives an intravenous infusion of mAb114--an experimental treatment for Ebola virus disease--in a Phase 1 clinical trial held at the NIH Clinical Center in Bethesda, Md.
NIAID
The investigational treatment is currently being offered to Ebola patients in the Democratic Republic of the Congo (DRC) under compassionate use and as part of a Phase 2/3 clinical trial of multiple investigational treatments. mAb114, a single monoclonal antibody, binds to the core receptor binding domain of the Zaire ebolavirus surface protein, preventing the virus from infecting human cells. Scientists isolated the antibody from a human survivor of the 1995 Ebola outbreak in Kikwit, DRC. Prior studies showed that mAb114 can protect monkeys from lethal Ebola virus disease when given as late as five days after infection.
Participants in the Phase 1 clinical trial received a single intravenous infusion of mAb114, administered over approximately 30 minutes. Three participants received a 5 milligram(mg)/kilogram (kg) dose; five participants received a 25 mg/kg dose; and 10 participants received a 50 mg/kg dose. All infusions were well-tolerated. Four participants reported mild side effects, such as discomfort, muscle or joint pain, headache, nausea, and chills in the three days following the infusion.
As expected, levels of mAb114 in the blood increased as the dosage was increased. Investigators also observed relatively uniform levels of absorption, distribution, and elimination of mAb114 among participants.
The authors note several advantages for deploying mAb114 in an outbreak setting, including the ease and speed of its administration, and its formulation as a freeze-dried powder that does not require freezer storage. The powder is reconstituted with sterile water and added to saline for administration.
In addition to the ongoing Phase 2/3 clinical trial of mAb114 in the DRC, the VRC is planning to initiate another Phase 1 trial of the investigational treatment in Africa.
Original publication
Martin R Gaudinski et al.; "Safety, tolerability, pharmacokinetics, and immunogenicity of the therapeutic monoclonal antibody mAb114 targeting Ebola virus glycoprotein (VRC 608): an open-label phase 1 study"; The Lancet; 2019
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic world Antibodies
Antibodies are specialized molecules of our immune system that can specifically recognize and neutralize pathogens or foreign substances. Antibody research in biotech and pharma has recognized this natural defense potential and is working intensively to make it therapeutically useful. From monoclonal antibodies used against cancer or autoimmune diseases to antibody-drug conjugates that specifically transport drugs to disease cells - the possibilities are enormous

Topic world Antibodies
Antibodies are specialized molecules of our immune system that can specifically recognize and neutralize pathogens or foreign substances. Antibody research in biotech and pharma has recognized this natural defense potential and is working intensively to make it therapeutically useful. From monoclonal antibodies used against cancer or autoimmune diseases to antibody-drug conjugates that specifically transport drugs to disease cells - the possibilities are enormous