Why nerve cells die
For many years researchers have observed protein deposits, also called aggregates, in the brains of patients with Alzheimer’s or Huntington’s disease. These aggregates are suspected to contribute to the death of nerve cells. As Science reports, researcher from the Max Planck Institute of Biochemistry in Martinsried, led by Mark Hipp and Ulrich Hartl, have now shown that the location of aggregates influences the survival of cells. While aggregates within the nucleus barely influence cellular function, deposits within the cytoplasm interfere with transport routes between the nucleus and the cytoplasm. In the long run this can lead to the death of the cells, and progression of the disease.
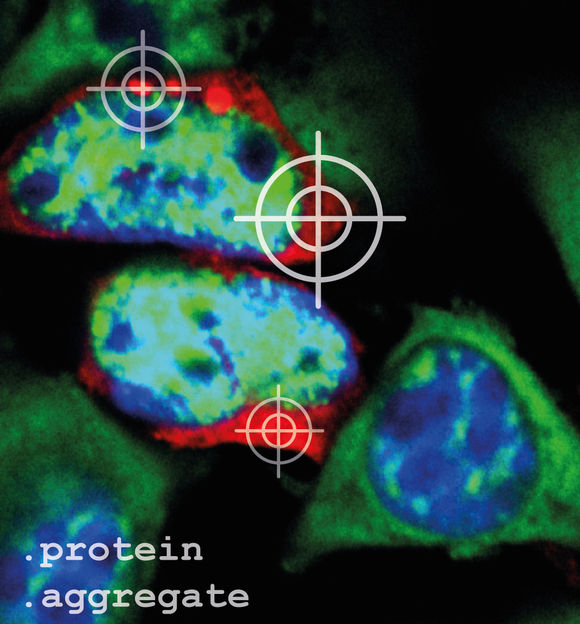
To visualize protein aggregates (red) under the microscope, they have to be stained. The cellular nucleus was stained blue and the mRNA, the construction manual for proteins, was stained green.
Andreas Woerner © MPI of Biochemistry
Proteins consist of long chains of amino acids and function in cells like small machines. To be able to fulfill their function proteins have to assume a predetermined three-dimensional structure. In healthy cells there is a large variety of folding helpers and extensive quality control machinery. Misfolded proteins are either repaired or rapidly degraded. If this occurs inadequately, or not at all, proteins will clump together, form aggregates and harm the cell.
Protein aggregates are associated with many neurodegenerative diseases including ALS, Alzheimer’s, Parkinson’s and Huntington’s Disease. How exactly aggregates harm cells is however still unknown. In 2013 several groups in Martinsried formed the ToPAG consortium to address this question, and can now report their first success. Scientists in the lab of Prof. Hartl have demonstrated that the location of the aggregates determines the fate of the nerve cells.
Together with Konstanze Winklhofer and Jörg Tatzelt from the Ruhr-University Bochum, the researchers have expressed artificial aggregation prone proteins as well as Huntington’s disease-causing mutants of the protein huntingtin in cultured cells. Both types of protein accumulate in large protein deposits. “It came as a big surprise to us that the direction of the proteins to the cytoplasm instead of the nucleus resulted in more soluble, but also more toxic aggregates”, says Mark Hipp, a group leader in the department of Ulrich Hartl and leader of the study. The protein deposits in the cytoplasm blocked the transport of RNA and correctly folded proteins between the nucleus and the cytoplasm. It seems that the sticky surfaces of the aggregates can sequester important proteins and thereby inactivate them. “We have detected multiple components of the cellular transport machinery inside the aggregates. This results in the depletion of these factors from the cell, and, like a machine with missing parts, the cell is then unable to function properly”, explains Andreas Woerner the first author of the study. Once the blueprint for all proteins, the RNA, is trapped within the nucleus, protein synthesis cannot progress, and the cells deteriorate. It is not completely clear why the nuclear aggregates are less harmful, but the researchers have evidence that the nuclear protein NPM1 plays a central role in shielding these aggregates.
“The results of this study bring us researchers and physicians one big step further”, summarizes Mark Hipp. “Only if we understand how aggregates damage cells is it possible to develop appropriate countermeasures in the future.”
Original publication
Original publication
A. C. Woerner, F. Frottin, D. Hornburg, L. R. Feng, F. Meissner, M. Patra, J. Tatzelt, M. Mann, K. F. Winklhofer, U. Hartl, M. S. Hipp: "Cytoplasmic protein aggregates interfere with nucleo-cytoplasmic transport of protein and RNA"; Science, 8. Januar 2016
Topics
Organizations
Other news from the department science

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
More news from our other portals
Last viewed contents

New procedure for producing safe and more effective vaccines
Lymphoma overrides a key protein's quadruple locks
How drugs get into the blood - This knowledge facilitates the development of drugs that can also be taken in tablet form

Researchers decode new antibiotic - Combined attack minimizes resistance development
Dynamic images show rhomboid protease in action

Those who live longer have fewer children - The time between two generations sets the price of fertility

World record broken for thinnest X-ray detector ever created - Highly sensitive and with a rapid response time, the new X-ray detector is less than 10 nanometres thick and could one day lead to real-time imaging of cellular biology
Intercell announces initiation in the U.S. of a Phase III study for vaccine to protect children against Japanese Encephalitis
Chinese scientists successfully crack the genome of diploid cotton

BASF to invest USD 33 million in R&D facilities in Research Triangle Park - Focus on plant biotechnology and crop protection research