'Metal' drugs to fight cancer
Advertisement
Pharmaceutical research can be difficult and frustrating. Often, one happens to synthesize a molecule without knowing exactly what kind of therapeutic effect it will have (if it ever will have any). "It is rare for someone to develop a new active drug already knowing what mechanism it will trigger in the body", explains Alessandra Magistrato, CNR-IOM/SISSA research scientist. "This also applies to the most widespread chemotherapeutic drugs, like cisplatin, or novel ones based on ruthenium". "Studies relying on modelling and simulations, like the ones we do here, may be very helpful in this sense, in that they increase our insights into the molecular mechanisms of action exerted by the drugs inside the body's cells", the scientist explains.
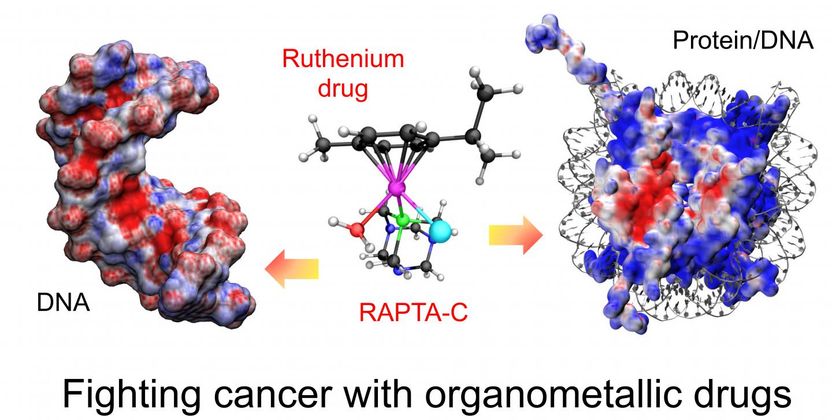
DNA and drug
Giulia Palermo
Magistrato is among the authors of a new study that reviews previously published experimental and computational reports visualized through the "lens" of computational microscope. "We produced models that enable us to rationalize the action of chemotherapeutic molecules on the body's cells", Magistrato explains. "For some types of drugs, we tried to understand which chemical form of the drug is most abundant when it enters the blood circulation and it reaches the cell ".
Scientists in fact use the term "prodrug" when referring to an injected chemotherapy agent; this, because as soon as the agent enters the body, it quickly changes before the interactions with it biological target. That's why it is difficult to know precisely which molecule (and how much of it) is responsible for the therapeutic action, in other words the actual medication.
On the other hand for other drugs with a known active form, Giulia Palermo, first author and researcher at the Swiss Federal Institute of Technology in Lausanne (EPFL), described how the the drug binds to different targets inside the cell. "A molecule can act on three fronts: on free DNA, on chromatin (the most common form of packed DNA in the nucleus) and on other proteins found in the cell", explains Palermo. Depending on which target is involved, the action of the drug can vary widely, as well as its side effects. "In fact, it is believed that when the drug exhibits cytotoxic effects, it may bound preferentially to the DNA, whereas when it has anti-metastatic effects, it may act on the proteins involved in motility or on protein/DNA complexes affecting gene regulation, for example".
"With the help of studies like this, experimentalists can improve the rational design of the new therapeutic molecules so as to obtain drugs that are more effective and with fewer side effects, a very important aspect as we know very well how physically demanding chemotherapy is for patients", concludes Magistrato.
The study is the result of an international collaboration between CNR-IOM/SISSA and the research groups led by Ursula Roethlisberger (professor of computational chemistry and biochemistry at EPFL), Paul Dyson, expert in organometallic and medicinal chemistry at EPFL, and Curt Davey, leader in crystallography of protein/DNA complexes at Nanyang Technological University (NTU) in Singapore.