Architecture of mTOR Protein Complex Solved
For a long time it has been known that the protein TOR – Target of Rapamycin – controls cell growth and is involved in the development of diseases such as cancer and diabetes. Researchers at the University of Basel’s Biozentrum together with scientists from ETH Zurich have now examined the structure of mammalian TOR complex 1 (mTORC1) in more detail.
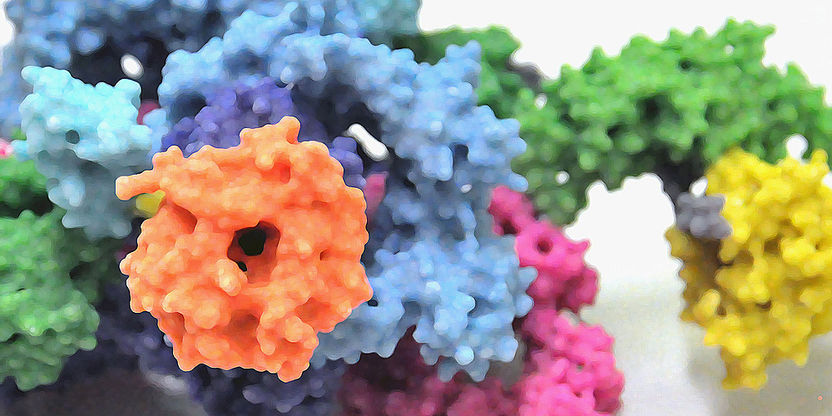
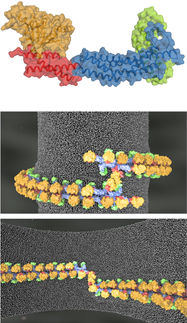
3D model of the protein complex mTORC1.
University of Basel, Biozentrum
About 25 years ago, Prof. Michael Hall discovered the protein “Target of Rapamycin” (TOR) at the Biozentrum. It is one of the most studied proteins of the protein kinase family, an important family of regulatory proteins that control many cellular processes. TOR, in mammals called mTOR, is very important for cellular signalling and is implicated in various diseases such as cancer, diabetes, and neurodegeneration. Several mTOR inhibitors have already been approved for therapeutic use, in particular in the treatment of cancer and allograft rejection.
However, despite extensive research on TOR over the last decades, attempts to uncover the detailed structure of the protein kinase and its partners have been unsuccessful. By combining crystallographic methods with cryo-electron microscopy, Prof. Timm Maier’s team together with researchers of the ETH Zurich have now been able to provide unprecedented insight into the architecture of the protein complex mTORC1.
Structure of mTORC1 elucidated
In the cell, the protein kinase mTOR is found in two structurally and functionally distinct protein complexes termed mTORC1 and mTORC2 in mammals. Both complexes are giant protein structures consisting of mTOR and other accompanying proteins. In these two configurations the protein kinase carries out various functions such as the control of cell size and growth, as well as the regulation of metabolism and energy balance.
mTOR itself is one of the largest proteins in the cell and when combined with other proteins even larger. This makes it quite difficult to investigate its structure. “The partner proteins of mTOR have already been identified in earlier biochemical studies”, says Maier. “However, it has remained unclear how the proteins interact precisely.” After more than three years of work, the scientists led by Maier have succeeded in isolating mTORC1 in the quality required for high-resolution cryo-electron microscopy. Using X-ray crystallography they have also been able to determine the structure of the protein Raptor, the second major component of mTORC1.
Accompanying proteins important for function
"Although there is much known about mTORC1, our study revealed surprising new insight”, states Maier. "The architecture of this huge protein complex is quite exceptional. We could determine the precise interaction sites of the partner proteins and how they are arranged, and thus elucidate the function of the individual partners.” In fact, each protein plays an important role in the regulation of the activity of the entire complex and the intracellular signalling cascade.
More than the sum of its parts
With their study, the researchers have provided the basis for further investigations. Now the researchers will be able to investigate the function of each individual protein in the complex in more detail. “But it doesn’t make sense to examine the individual components alone, as the interactions of all the proteins in the complex are critical for its function”, explains Maier. “The whole is much more than the sum of its parts.” The finely tuned regulation of mTOR activity is very important because even the smallest disturbances can have serious consequences. Thus, dysregulation in the mTOR signalling pathways plays a role in the development of a number of diseases.
Original publication
Other news from the department science

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
More news from our other portals
Last viewed contents
Pharming completes spin-off of DNage
Pharmacelsus’ GLP implementation exemplary for European CROs
Demonic_Males:_Apes_and_the_Origins_of_Human_Violence
CSIRO Molecular and Health Technologies - Clayton North, Australia
LDC Receives 1 Million Euros from the Max Planck Foundation for the Development of New Drug Candidates
Team Led by Scripps Research Scientists Uncovers New Way to Limit Damaging Production of Nitric Oxide - Results Could Lead to New Treatments for Conditions From Inflammation to Cancer
Successful Pilot Study for Non-Invasive Prenatal Diagnostic Test to Determine Trisomy 21