Stratification of the severity of Non Alcoholic Fatty Liver disease
Advertisement
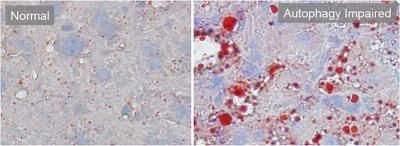
It has been estimated in Europe that approximately 26 % or higher health costs account for NAFLD patients. NAFLD is a typical metabolic disease associated with obesity, insulin resistance and type 2 diabetes. Abnormal fat deposits develop in patients’ livers. In general steatosis is without complications however associated with an increased risk of heart attacks and strokes. Furthermore, steatosis can progress to steatohepatitis and in some cases to cirrhosis and liver cancer. There are also genetic mutations associations with the disease, for example within genes such as PNPLA3 and TM6SF2. Taken together, NAFLD is caused by a complex interplay of genetic and environmental factors.
Therefore to gain insights into disease causing mechanisms James Adjaye co-ordinated the livSYSiPS consortium, which was co-funded by the German Federal Ministry of Education and Research (BMBF), a partner of the ERASysBio+ initiative supported under the EU ERA-NET Plus scheme in FP7. The ERASysBio+ initiative aimed at advancing the progress of systems biology research in Europe. The multi-national consortium with research groups from Germany, UK, Italy and Austria employed a systems biology approach to investigate the etiology of NAFLD.
The underlying study discovered higher amounts of the gene PLIN2 in NAFLD patients. PLIN2 is involved in the aggregation of the fat deposits in the liver. Mice without this protein do not become fat even when overfed with a high-fat diet. Additional findings in blood serum of these patients include differences in the levels of the appetite-controlling hormone LEPTIN, ADIPONECTIN and amino acids which control the break-down of fat. Furthermore, changes in the complex signaling cascades of insulin were detected which is the main regulation mechanism of glucose and fat balance. These distinctions are an encouraging basis for further research encompassing a much larger cohort of patients with the potential to find novel means for diagnosing NAFLD and eventually new therapies.
Essential for this goal is good quality systems biology-based datasets and models and the sharing of data between researchers to catalyze scientific progress. Wasco Wruck, Bioinformatician and first author of the study, says: ”From our multi-disciplinary measurements we got amazing datasets from which we could draw interesting findings and hypothesis about NAFLD. We need further patient cohorts and experiments to confirm our initial results and to improve our systems biology models. In order to allow other researchers to work on this valuable data we deposited it in public repositories.”
To gain further insights to the cause of the disease at the level of individuals, which is the new era of personalized medicine, James Adjaye’s team are now generating induced pluripotent stem cells (iPSCs) from skin cells from these patients and matching healthy controls. These patient-specific iPSCs are then turned into hepatocytes which are cells within the liver with the view of mimicking the patient’s liver metabolism in a dish. To induce steatosis we add excess fat (e.g. oleic acid) to these cells and then analyse changes in gene, protein and metabolite levels as we originally did with the original liver biopsy and blood serum samples. Our goal now is to see if our “disease in a dish” model mirrors that seen in the patient, define new biomarkers of NAFLD and identify pathways as putative drug targets that can be used to complement current therapies for NAFLD says James Adjaye who is the senior author of the current publication.
Original publication
Wasco Wruck, Karl Kashofer, Samrina Rehman, Andriani Daskalaki, Daniela Berg, Ewa Gralka, Justyna Jozefczuk, Katharina Drews, Vikash Pandey, Christian Regenbrecht, Christoph Wierling, Paola Turano, Ulrike Korf, Kurt Zatloukal, Hans Lehrach, Hans Westerhoff, and James Adjaye; "Multi-omic profiles of human non-alcoholic fatty liver disease tissue highlight heterogenic phenotypes"; Scientific Data; 2015