Scripps Florida scientists create 'fingerprints' for major drug development targets
For the first time, scientists from the Florida campus of The Scripps Research Institute (TSRI) have created detailed "fingerprints" of a class of surface receptors that have proven highly useful for drug development.
These detailed "fingerprints" show the surprising complexity of how these receptors activate their binding partners to produce a wide range of signaling actions.
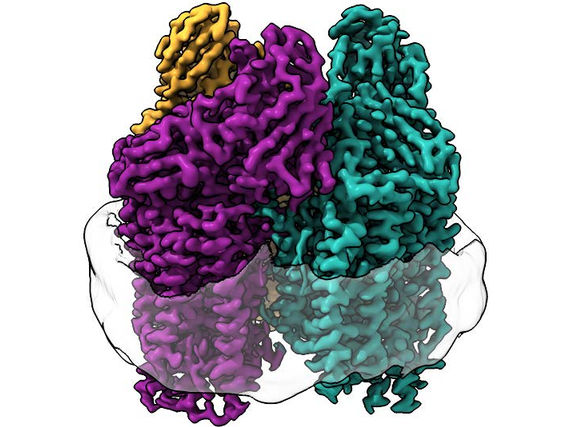
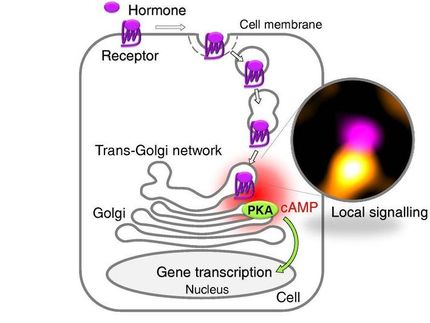
The study focuses on interactions of G protein-coupled receptors (GPCRs) with their signaling mediators known as G proteins. GPCRs--currently accounting for about 40 percent of all prescription pharmaceuticals on the market--play key roles in many physiological functions because they transmit signals from outside the cell to the interior. When an outside substance binds to a GPCR, it activates a G protein inside the cell to release components and create a specific cellular response.
"Until now, it was generally believed that GPCRs are very selective, only activating few G proteins they were designed to work with," said TSRI Associate Professor Kirill Martemyanov, who led the study. "It turns out the reality is much more complex."
Ikuo Masuho, a senior research associate in the Martemyanov lab, added, "Our imaging technology opens a unique avenue of developing drugs that would precisely control complex GPCR-G protein coupling, maximizing therapeutic potency by activating G proteins that contribute to therapeutic efficacy while inhibiting other G proteins that cause adverse side effects."
The study found that individual GPCRs engage multiple G proteins with varying efficacy and rates, much like a dance where the most desirable partner, the GPCR, is surrounded by 14 suitors all vying for attention. The results, as in any dance, depend on which G proteins bind to the receptor--and for how long. The same receptor changes G protein partners--and the signaling outcome--depending on the action of the signal received from outside of the cell.
This finding was made possible by novel imaging technology used by the Martemyanov lab to monitor G protein activation in live cells. Using a pair of light-emitting proteins, one attached to the G protein, the other attached to what's known as a reporter molecule, Martemyanov and his colleagues were able to measure simultaneously both the signal and activation rates of most G proteins present in the body.
"Our approach looks at 14 different types of G proteins at once--and we only have 16 in our bodies," he said. "This is as close as it can get to what is actually happening in real time."
Original publication
Most read news
Original publication
Masuho, Ikuo and Ostrovskaya, Olga and Kramer, Grant M. and Jones, Christopher D. and Xie, Keqiang and Martemyanov, Kirill A.; "Distinct profiles of functional discrimination among G proteins determine the actions of G protein–coupled receptors"; Science Signaling; 2015
Organizations
Other news from the department science

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Friedrich_Schultze