Uptake mechanisms of cytostatics discovered
Advertisement
How does a cytostatic like cisplatin or carboplatin actually get into the cell? Scientists at the MaxDelbrück Center for Molecular Medicine in the Helmholtz Association (MDC) and the Leibniz-Institut für Molekulare Pharmakologie (FMP) in Berlin, in cooperation with a Dutch group, have now succeeded in showing that the volume-regulated anion channel VRAC is 50 % responsible for active substance uptake. If one of the VRAC subunits LRRC8A or LRRC8D is down-regulated, cells take up considerably less of the anti-cancer drug. In addition to this finding, programmed cell death or apoptosis is also significantly disturbed when LRRC8A is missing. The researchers have thus identified a potential cause for therapy resistance.
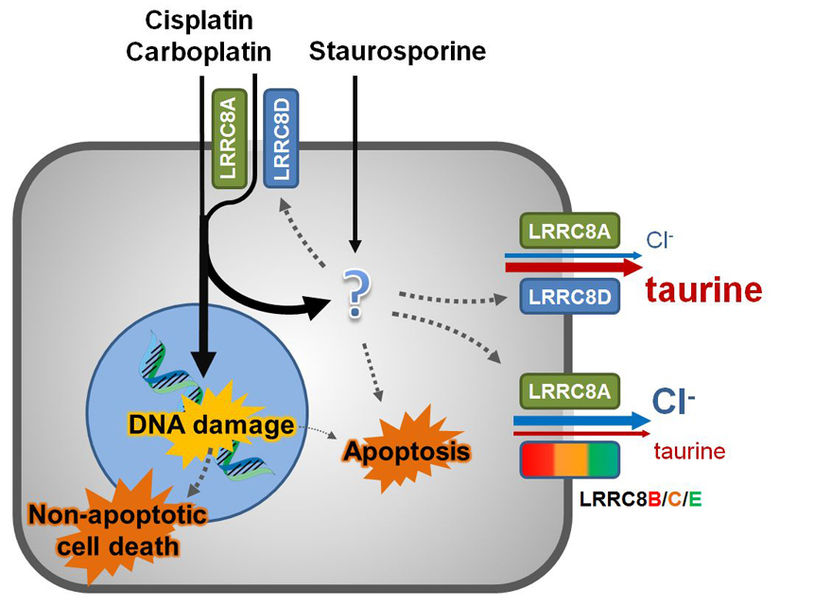
The anti-cancer drugs cisplatin and carboplatin and the pro-apoptotic staurosporine enter cells through passive diffusion. Pt-based drugs induce non-apoptotic cell death, and to a lesser extent, apoptosis through DNA damage, while both Pt-based drugs and staurosporine can induce apoptosis through an additional, poorly characterized pathway. VRACs are also activated by pro-apoptotic stimuli. Channels containing LRRC8A and LRRC8D mainly transport osmolytes like taurine, but also provide a further pathway for uptake of Pt-based drugs, but not for the larger staurosporine. Thus, Pt-based drugs promote their own uptake through a feed-forward style mechanism.
Thomas Jentsch, FMP/MDC
A good 18 months ago, researchers at the MaxDelbrück Center for Molecular Medicine (MDC) and the Leibniz-Institut für Molekulare Pharmakologie (FMP) discovered the molecular identity of VRAC. VRAC is a volume-regulated anion channel that allows negatively charged ions (anions) and amino acids into the cell and back out again. VRAC, which is formed by the protein LRRC8A and at least one relative, is also believed to play a role in cell division and cancer.
VRAC is decisive for active substance uptake
In collaboration with Dutch colleagues, the authors now show how important VRAC is, particularly in cancer. They investigated cell lines to determine the role played by VRAC and its subunits in the transport of cisplatin and carboplatin into the cell. The researchers themselves were surprised by the result: VRAC or rather its protein subunits LRRC8A and LRRC8D are responsible for half of the uptake of two widely used cytostatics. In other words: Without these two subunits, only little active substance finds its way into the cell. From the researchers' point of view, this goes some way into explaining why some people are resistant to some forms of cancer therapy.
In their experiments, the work group of Thomas Jentsch had systematically switched off different combinations of VRAC forming proteins. When it was the turn of LRRC8A and LRRC8D, the cells were barely able to take up any of the anti-cancer drugs. "For a long time, there has indeed been a hypothesis that VRAC plays a decisive role in apoptosis. But the discovery that the pressure valve also serves as an uptake mechanism for cytostatics was a real surprise," says Thomas Jentsch.
Impaired apoptosis increases therapy resistance
The recent study also confirmed the hypothesis concerning apoptosis. If the protein LRRC8A, vital for VRAC, was put out of action, natural cell death no longer functioned properly.
According to the researchers, the apoptotic process is to be seen as completely independent of the uptake mechanism. Jentsch speaks of a double mechanism. The Berlin-based ion-channel researcher stresses: "The suppression of apoptosis is probably due to the fact that, in the absence of volume-regulating VRAC, the cell shrinkage observed in programmed cell death no longer functions. This has nothing to do with the mechanism of medication uptake."
This novel uptake mechanism could even be confirmed by clinical data in this study. Researchers led by Sven Rottenberg of the Cancer Research Centre in Amsterdam also identified LRRC8D as a relevant gene in a genome-wide screen for cellular cytostatic resistance. They studied the genetic data of ovarian cancer patients, who had been treated with cisplatin or carboplatin, with regard to their survival time. Tumour database analysis showed that reduced LRRC8D expression correlated with a decreased survival time in these patients.
Results with clinical relevance
So, does the absence of the VRAC protein promote therapy resistances? "The data suggest that LRRC8A and LRRC8D are also clinically relevant resistance genes, although this finding still has to be substantiated through prospective studies," says basic researcher Jentsch. And what next? “Purely theoretically, it might be possible to find activators, in order to re-activate the pressure valve,” believes Jentsch. The search is already underway in the Screening Unit at the FMP.
Alongside the two clinically relevant mechanisms, the study also unearthed a previously unknown physiological mechanism. Apparently, the VRAC subunit LRRC8D is immensely important for the transport of the amino acid taurine, which plays an important role as an organic osmolyte in volume regulation, but also stimulates important receptors in the brain. By switching off LRRC8D, it will now be possible to specifically investigate physiological and pathological roles of taurine release by VRAC. As a whole, the study has demonstrated once more how quickly basic research can lead to clinically important insights.
Original publication
R. Planells‐Cases, D. Lutter, C. Guyader, N. M. Gerhards, F. Ullrich, D. A. Elger, A. Kucukosmanoglu, G. Xu, F. K. Voss, S. Momsen Reincke, T. Stauber, V. A. Blomen, D. J. Vis, L. F. Wessels, T. R. Brummelkamp, P. Borst, S. Rottenberg, T. J. Jentsch; "Subunit composition of VRAC channels determines substrate specificity and cellular resistance to Pt‐based anti‐cancer drugs"; EMBO; 2015