Alzheimer’s Disease: New Test Identifies the Most Promising Drug Candidates
Despite enormous efforts of scientists around the world, an effective and causal treatment against Alzheimer’s disease is yet to be found. Many substances that seemed promising initially, later failed in clinical trials, proving ineffective only after years of costly and time consuming tests. This may be due to the wrong molecular targets of these substances and the lack of accurate analytical methods to assess their efficacies. Scientists from Forschungszentrum Jülich and Heinrich Heine University Düsseldorf now have developed QIAD, an in vitro method that could make it easier to earlier identify the most promising substances. The assay measures the effect of compounds on the size distribution of toxic protein aggregates with previously unavailable precision. In animal studies, these measurements proved to be predictive for therapeutic efficacy. In the future, QIAD could help researchers avoid unnecessary animal studies with ineffective compounds by allowing efficient pre-selection.
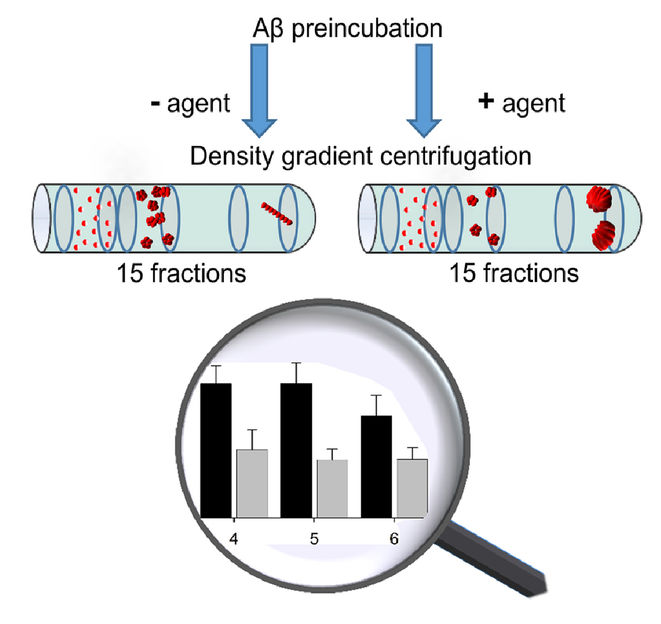
The QIAD assay (right) determines how a therapeutic agent affects the aggregate size distribution.
Copyright: Forschungszentrum Jülich / HHU
In Alzheimer’s disease, patients suffer a massive loss of nerve cells in the brain leading to a severe decline in cognitive ability. The aggregation of the endogenous protein amyloid-beta (Aβ) is thought to be decisive for disease development. Single molecules, so called monomers, of Aβ bind to each other to eventually form aggregates and large insoluble fibrils and plaques, which are visible as deposits between the nerve cells in the brain. Many drug candidates have targeted these aggregates. However, new research has shown that the aggregates toxicity greatly varies with size and fibrils and plaques may not represent the most harmful forms. Instead, a growing body of evidence now indicates that small, freely moving Aβ aggregates, called Aβ oligomers, are key drivers in the development and progression of the disease, with a particularly toxic effect on nerve cells.
"With this, the ability to eliminate these Aβ-oligomers has emerged as one of the most desirable properties to look for in a drug candidate against Alzheimer’s disease", says Prof. Dieter Willbold, director at both the Institute of Complex Systems: Structural Biochemistry (ICS-6) at Forschungszentrum Jülich and the Institut für Physikalische Biologie at Heinrich Heine University Düsseldorf. Whether or not a substance eliminates these specific aggregate sizes in principle could be assessed as a predictor of therapeutic efficacy prior to time consuming animal studies. So far however, a suitable method for this has been missing.
"The lack of a quantitative assay became apparent to us during our own drug development research", the scientist explains. Over the past several years, Willbold and his teams in Jülich and Düsseldorf have been developing a new oligomer-targeting drug candidate called "D3". In previous studies, treatment with D3 led to significant improvements in mice which exhibit similar amyloid deposits as human patients and suffer from cognitive deficits. Since then, the scientists have worked on optimized derivatives and are currently preparing the first-in-man study. "In this optimization stage we wanted a way to precisely track how different variations of the compound affect different aggregate sizes", Willbold says.
To this end, first author Dr. Oleksandr Brener, then a doctoral student of Willbold, in close collaboration with other scientists of the institute developed a new method called QIAD (quantitative determination of interference with Aβ aggregate size distribution). Through a special centrifugation technique, protein molecules and aggregates of different sizes in a sample are first sorted by size into individual fractions. After that, each fraction can be quantitatively analyzed, in this case, a technique called reversed phase high performance liquid chromatography was used. By comparing samples with and without the added drug candidate, the effects on different aggregate sizes can be measured simultaneously and quantitatively. To validate the reliability of this method, it was applied to a number of known drug candidates which had been extensively tested. The QIAD outcomes matched the previously reported preclinical and clinical results of these studies.
To see if the measurements could furthermore be used to predict therapeutic efficacy in an organism, the scientist then compared the original version of their own drug candidate D3 and the optimized derivative “D3D3”, which was expected to eliminate oligomers more efficiently. After the measurements had indeed shown that D3D3 eliminated 96 percent of oligomers from the sample, compared to approximately 50 percent for the original version, the effects of both compounds were tested in mouse models. As predicted from the QIAD outcomes, D3D3 slowed neurodegeneration in the mice’s brain much more effectively, as Dr. Tina Dunkelmann was able to show during her dissertation. Collaborators Dr. Thomas van Groen and Dr. Inga Kadish at the University of Alabama at Birmingham furthermore showed that D3D3 also improves cognition.
“This has a twofold significance for us”, Willbold explains. “It provided important information for the ongoing optimization of our drug candidate. At the same time it showed that the QIAD assay can indeed predict a compound’s therapeutic efficacy.” In the future, QIAD could guide drug developers in picking the most promising drug candidates for further testing, thereby reducing the high number of clinical failures in Alzheimer’s research. Furthermore, since aggregates of endogenous proteins also play an important role in Parkinson’s and numerous other neurodegenerative diseases, the assay could be adapted for drug research in these areas too.
Original publication
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic world HPLC
HPLC is a key technology in modern analytical chemistry. It enables the separation, identification and quantification of components in complex mixtures with high precision and efficiency. Whether in the analysis of active pharmaceutical ingredients, the quality control of foodstuffs or the examination of biological samples - HPLC is often the method of choice for demanding separation tasks.

Topic world HPLC
HPLC is a key technology in modern analytical chemistry. It enables the separation, identification and quantification of components in complex mixtures with high precision and efficiency. Whether in the analysis of active pharmaceutical ingredients, the quality control of foodstuffs or the examination of biological samples - HPLC is often the method of choice for demanding separation tasks.