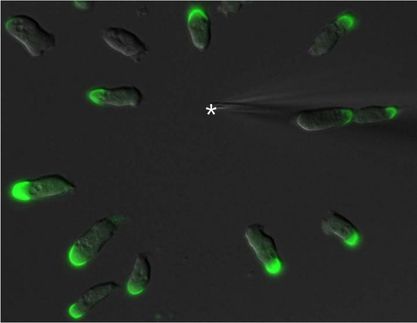
The Solution to a 50-Year-Old Riddle: why Certain Cells Repel one Another
When cells from the connective tissue collide, they repel one another – this phenomenon was discovered more than 50 years ago. It is only now, however, that researchers at the University of Basel have discovered the molecular basis for this process. Their findings could have important implications for cancer research.
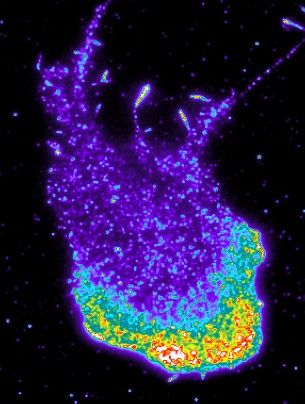
A motile fibroblast: the protein srGAP2, which initiates the repulsion reaction, is heavily concentrated at the front of the cell (below, in red, yellow, and green).
© University of Basel, Prof. Pertz’s research group
Fibroblasts are motile constituents of the connective tissue and also regulate its stiffness. Moreover, fibroblasts play an important role in malignant skin diseases such as melanoma. In research, they serve as a model system for studying cell migration.
Signaling pathway identified
In the early 1950s, the English researcher Michael Abercrombie discovered that colliding fibroblasts repel one another and, in the process, change their direction of motion. He called this phenomenon ‘contact inhibition of locomotion’. Although individual proteins were identified as key factors in this process, the molecular basis of this reaction remained something of a puzzle. In particular, it was unclear which repulsion signals were involved in the process, how these signals entered the cells from the outside, and how they influenced the cytoskeleton, which in turn regulates the cell’s movement.
Prof. Olivier Pertz’s research group at the University of Basel has now precisely answered these questions. The group identified a coherent signaling axis consisting of three proteins called Slit2, Robo4, and srGAP2 which operates as follows:
- The repulsion factor Slit2 binds to the receptor Robo4, whereupon the signal enters the cell’s interior and activates srGAP2.
- This molecule consequently inhibits the regulator Rac1, which coordinates the cytoskeleton.
- The inactivation of Rac1 causes the cell to retract – such that the two cells repel one another.
If the function of Slit2, Robo4, or srGAP2 is deactivated, colliding cells will stick to one another and will not separate as easily.
A ‘molecular bumper’
Intriguingly, the repulsion machinery is localized at the front – even in freely moving cells. By assembling this kind of a ‘molecular bumper’, the cell is prepared for collision with another cell. Where exactly this bumper must be positioned – namely, only in parts of the cell that are moving forwards – is determined by the cell’s geometry, which in turn is deciphered by srGAP2.
The integration of membrane curvature and repulsion signals ensures that cell-cell repulsion takes place at the correct location. This repulsive reaction could play an important role in cancer metastasis. This is supported by the fact that the expression of Slit and Robo isoforms is deregulated in several tumor types.
Original publication
Rafael Dominik Fritz, Denis Menshykau, Katrin Martin, Andreas Reimann, Valeria Pontelli, and Olivier Pertz; SrGAP2-Dependent Integration of Membrane Geometry and Slit-Robo-Repulsive Cues Regulates Fibroblast Contact Inhibition of Locomotion; Developmental Cell (2015)
Original publication
Rafael Dominik Fritz, Denis Menshykau, Katrin Martin, Andreas Reimann, Valeria Pontelli, and Olivier Pertz; SrGAP2-Dependent Integration of Membrane Geometry and Slit-Robo-Repulsive Cues Regulates Fibroblast Contact Inhibition of Locomotion; Developmental Cell (2015)
Organizations
Other news from the department science

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.