Molecular 'kiss of death' flags pathogens
Many bugs that make us sick hide out in our cells in protective little bubbles called vacuoles. To clear an infection, the immune system must recognize and destroy these vacuoles while leaving the rest of the living cell intact.
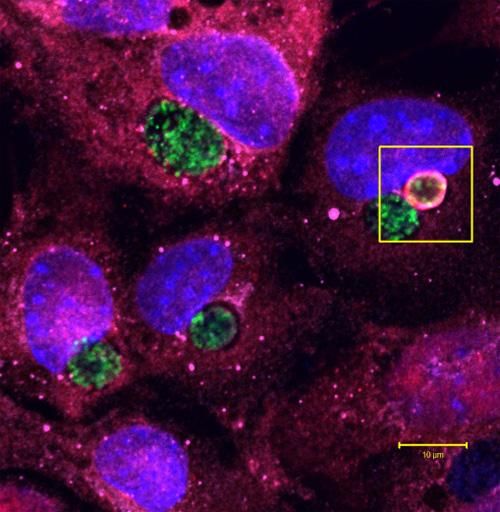
The body uses a molecule called ubiquitin, here shown in red inside the box, to tag little pockets of pathogen, called vacuoles, as they bloom on the surface of a cell. The vacuole in this image is full of Chlamydia trachomatis (shown in green), the bacteria which cause a common sexually transmitted infection, but the ubiquitin molecules have marked the vacuole for destruction by the immune system.
Dr. Arun Haldar
Now, researchers have discovered that our bodies mark pathogen-containing vacuoles for destruction by using a molecule called ubiquitin, commonly known as the "kiss of death."
The finding could lead to new therapeutic strategies to boost the immune system's response to the pathogens responsible for a long list of human ailments, including tuberculosis, salmonella, chlamydia, toxoplasmosis and malaria.
"To get rid of these pathogens, the immune system essentially has to find a needle in a haystack," said Jörn Coers, Ph.D., assistant professor of molecular genetics and microbiology at Duke University School of Medicine. "It has to target a single microbe, in a vacuole, in an ocean of other membranes, floating around inside the cell. We found that the immune system accomplishes this feat by painting the vacuole with a coat of ubiquitin, which allows for the recruitment of all these other factors that viciously attack the vacuole and eliminate the pathogen inside."
When pathogens first enter a host cell, they take part of the plasma membrane with them, wrapping it around themselves like a cloak to mask their true identity. Eventually, a healthy immune system discovers the invasion and puts special molecules called guanylate binding proteins (GBPs) on high alert. These proteins specifically bind to the membranes of the pathogen-containing vacuoles and eliminate the infiltrators. Coers and his colleagues wanted to figure out how the GBPs know which membrane-bound structures to go after.
The researchers first conducted a large-scale screen for proteins involved in the clearance of pathogens. To their surprise, their search turned up a few proteins that play a role in ubiquitination, the process whereby the body's own doomed proteins are marked for destruction with the addition of ubiquitin tags. No previous studies had ever drawn a connection between ubiquitin and the destruction and elimination of pathogen-containing vacuoles by GBPs.
Coers and his colleagues decided to look for these ubiquitin tags on vacuoles containing two different microorganisms: Chlamydia trachomatis, the causative agent of the most common sexually transmitted bacterial infection, and Toxoplasma gondii, the single-celled parasite that causes toxoplasmosis.
First, they "primed" the cells with cytokines, the signaling molecules that kick the immune system into action. Then, they stained the cells with a red dye that was specific for the ubiquitin protein.
"All of a sudden we saw these beautiful rings of ubiquitin that nicely decorated the outsides of the pathogen-containing vacuoles," said Coers. He and his colleagues went on to identify the molecular players responsible both for attaching the ubiquitin tags and for escorting the GBPs to the surface of the vacuole so they can coordinate an attack.
The researchers also showed that highly virulent strains of Chlamydia and Toxoplasma contain special factors that block the addition of these ubiquitin tags. Because their vacuoles don't get ubiquitinated, the GBPs fail to recognize them as the enemy.
In the future, the researchers would like to determine what other tricks pathogens use to evade the immune response. Once they have a clear idea of what makes some of these pathogens more dangerous, they can design therapeutics to render these hypervirulent strains more susceptible to the host response.