Chemists solve major piece of cellular mystery
Team determines the architecture of a second subcomplex of the nuclear pore complex
Not just anything is allowed to enter the nucleus, a double membrane serves as a wall, protecting the contents of the nucleus. Any molecules trying to enter or exit the nucleus must do so via a cellular gatekeeper known as the nuclear pore complex (NPC).
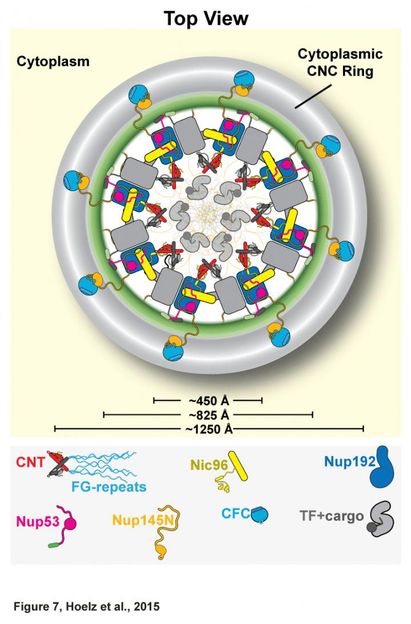
This is the architecture of the inner ring of the NPC proposed by the Caltech-led team. The channel nuceloporin complex (labeled here as CNT) is attached at only one site, the adaptor nucleoprin Nic96. This helps prove that the channel does not change shape or dilate.
Hoelz Laboratory/Caltech
How can the NPC be such an effective gatekeeper - preventing much from entering the nucleus while helping to shuttle certain molecules across the nuclear envelope? Scientists have been trying to figure that out for decades, at least in part because the NPC is targeted by a number of diseases, including some aggressive forms of leukemia and nervous system disorders such as a hereditary form of Lou Gehrig's disease. Now a team led by André Hoelz, assistant professor of biochemistry at Caltech, has solved a crucial piece of the puzzle.
Hoelz and his colleagues already described the atomic structure of the NPC's coat nucleoporin complex. Building on that work, the team has now solved the architecture of the pore's inner ring, a subcomplex that is central to the NPC's ability to serve as a barrier and transport facilitator. In order to the determine that architecture, which determines how the ring's proteins interact with each other, the biochemists built up the complex in a test tube and then systematically dissected it to understand the individual interactions between components. Then they validated that this is actually how it works in vivo, in a species of fungus.
For more than a decade, other researchers have suggested that the inner ring is highly flexible and expands to allow large macromolecules to pass through. "People have proposed some complicated models to explain how this might happen," says Hoelz. But now he and his colleagues have shown that these models are incorrect and that these dilations simply do not occur.
"Using an interdisciplinary approach, we solved the architecture of this subcomplex and showed that it cannot change shape significantly," says Hoelz. "It is a relatively rigid scaffold that is incorporated into the pore and basically just sits as a decoration, like pom-poms on a bicycle. It cannot dilate."
Together, the inner and outer rings make up the symmetric core of the NPC, a structure that includes 21 different proteins. The symmetric core is so named because of its radial symmetry. They started solving the architecture by focusing on the channel nucleoporin complex, or channel, which lines the central transport channel and is made up of three proteins, accounting for about half of the inner ring. This complex produces filamentous structures that serve as docking sites for specific proteins that ferry molecules across the nuclear envelope.
The biochemists employed bacteria to make the proteins associated with the inner ring in a test tube and mixed various combinations until they built the entire subcomplex. Once they had reconstituted the inner ring subcomplex, they were able to modify it to investigate how it is held together and which of its components are critical, and to determine how the channel is attached to the rest of the pore.
Hoelz and his team found that the channel is attached at only one site. This means that it cannot stretch significantly because such shape changes require multiple attachment points. When the researchers introduced mutations that effectively eliminated the channel's single attachment, the complex could no longer be incorporated into the inner ring. After proving this in the test tube, they also showed this to be true in living cells.
Original publication
Tobias Stuwe, Christopher J. Bley, Karsten Thierbach, Stefan Petrovic, Sandra Schilbach, Daniel J. Mayo, Thibaud Perriches, Emily J. Rundlet, Young E. Jeon, Leslie N. Collins, Ferdinand M. Huber, Daniel H. Lin, Marcin Paduch, Akiko Koide, Vincent Lu, Jessica Fischer, Ed Hurt, Shohei Koide, Anthony A. Kossiakoff, André Hoelz; "Architecture of the fungal nuclear pore inner ring complex"; Science; 2015



















































