New clues to the genetic origins of obesity
Like many other conditions, obesity is caused by an interplay between genetic and environmental factors. While efforts to combat the obesity epidemic will need to include changes in diet and exercise, insights into the genes involved may also help with prevention and treatment.
Now a research team led by investigators at Beth Israel Deaconess Medical Center (BIDMC) and the Massachusetts Institute of Technology (MIT) reveals the mechanistic explanation behind the strongest genetic association with obesity. The findings, published in the New England Journal of Medicine, uncover a genetic circuit that controls whether our bodies burn or store fat. Manipulating that genetic circuit may offer a new approach for obesity treatments.
The strongest genetic association with obesity lies within an unexpressed region of the FTO gene. The obesity-risk version of the region that predisposes individuals to increased body weight is found in 44 percent of individuals in European populations, but its mechanistic basis has until now remained unknown, despite extensive investigation.
To identify the cell types in which the FTO obesity-risk region might exert its effects, the researchers analyzed information from the Roadmap Epigenomics project, which assesses chemical or "epigenetic" modifications within chromosomes that switch genes on or off. The project's data revealed that the strongest epigenetic signal was found in "preadipocyte" cells, the progenitor cells that go on to become fat cells.
"Previous studies attempted to uncover a link between FTO and the regulation of appetite or propensity to exercise controlled by the brain," said the study's lead and corresponding author Melina Claussnitzer, PhD, a visiting professor at MIT's Computer Science and Artificial Intelligence Laboratory (CSAIL). "But an unbiased look across more than one hundred human tissues and cell types indicated that the obesity-associated region acts primarily in adipocyte progenitor cells - not the brain."
"Despite years of investigating the FTO obesity region, no substantial expression differences were found between obesity-risk and non-risk individuals in brain or other tissue types, making it difficult to trace its mechanism of action," said Manolis Kellis, PhD, professor at MIT's CSAIL. "We found a strong difference for both IRX3 and IRX5 in preadipocytes, revealing the target genes, cell type and developmental stage where the genetic variant acts, thus enabling us to begin dissecting its mechanism of action."
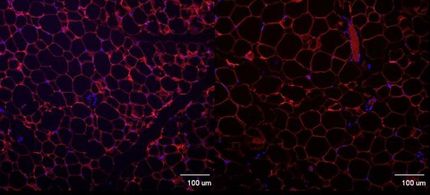
Elevated expression of these genes resulted in a shift from energy-burning beige fat cells to energy-storing white fat cells. The researchers showed that they could manipulate this new pathway to reverse the signatures of obesity. "By altering the expression of either gene in human preadipoctyes, we could alter adipocyte metabolism between energy storage and energy dissipation, providing a direct link between IRX3 and IRX5 expression and energy balance," said Kellis.
To evaluate the effect of IRX3 inhibition on whole-body energy metabolism and body weight, the team inhibited the corresponding gene in the fat cells of mice. The animals' metabolism increased and they lost weight, even though their physical activity and appetite were unchanged. "The results at the organism level were dramatic," said Claussnitzer. "These mice were 50 percent thinner than the control mice, and they did not gain any weight on a high-fat diet. Instead they dissipated more energy, even in their sleep, suggesting a dramatic shift in their global metabolism. The circuitry underlying the FTO region functions like a master regulatory switch between energy storage and energy dissipation."The researchers then sought to connect these differences in metabolism and gene expression to the genetic differences between lean and obese people within the FTO gene. They predicted that the specific T-to-C single-nucleotide alteration within FTO is responsible for the obesity association by repressing an evolutionarily-conserved gene regulator called ARID5B. Loss of repression turns on IRX3 and IRX5 during early adipocyte differentiation, leading to a shift from beige adipocyte functions and thermogenesis, or energy burning, to white adipocyte lipid accumulation.
Using the genome editing technique known as CRISPR/Cas9, the team found that switching the risk variant to the protective variant in preadipocytes turned off IRX3 and IRX5 and restored thermogenesis, while the reverse change turned on IRX3 and IRX5 and turned off thermogenesis.