Intersection between nervous and musculoskeletal system could be key to treating Pompe disease
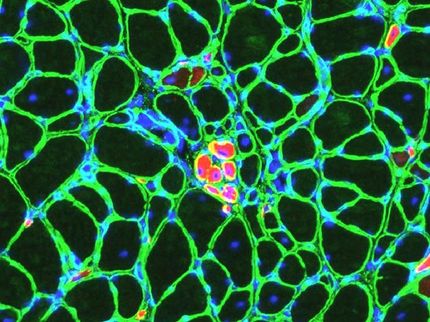
Walking and breathing without mechanical assistance typically becomes a struggle for patients who have Pompe disease because of weakened, damaged muscles as they age. Now, University of Florida Health researchers have identified how prior to these devastating effects, hidden physiological changes occur in the body’s neuromuscular junction, the part of the body where the nervous system connects to the musculoskeletal system.
These negative changes that occur in the neuromuscular junction trigger the muscle weakness that causes the mobility and breathing challenges these patients face, according to research the scientists recently presented at the American Society of Cell and Gene Therapy’s annual meeting.
With this understanding, UF Health researchers also have conducted early gene therapy studies in a Pompe disease mouse model using a virus delivery system that not only targets the muscle, but also reaches the nervous system. Early results in mice using gene therapy with adeno-associated virus 9, or AAV9 — which deftly shepherds a corrective gene into the body — show the therapy improved breathing function in diseased mice more than the current conventional therapy.
“Existing therapy is limited to treating striated muscle, and we do not see the improvement you would expect,” said Darin Falk, Ph.D., an assistant professor of pediatrics and a lead author of the studies. “While existing therapy provides some stabilization of function, there is limited improvement in some patients, which is why we looked at alternative explanations directly related to activation of muscle function. We believe the neuromuscular junction plays a major role contributing to weakness in Pompe disease.”
A rare form of muscular dystrophy and glycogen storage disease, Pompe disease occurs when a mutation develops on a gene instrumental to producing the enzyme acid alpha-glucosidase, or GAA. The GAA enzyme helps cells break down stored sugar called glycogen. Without this crucial enzyme, the glycogen builds up, leading to tissue and organ damage in the body. About 1 in 40,000 people have this inherited condition.
Currently, the only FDA-approved treatment for the disease is enzyme-replacement therapy.
“Although this therapy slows the disease, it does not stop its progression,” said Barry Byrne, M.D., Ph.D., a professor of pediatrics and molecular genetics and microbiology in the College of Medicine, director of the Powell Gene Therapy Center, a member of the UF Genetics Institute and the senior author of the studies.
The key to this treatment is the use of AAV9. Unlike other forms of AAV — long considered the gold standard virus to transport corrective genes into the body safely during gene therapy — AAV9 can spread more widely through the body, even penetrating the well-protected central nervous system.
In their study of a Pompe disease mouse model, the researchers treated one group of mice with AAV9 gene therapy and another with the standard enzyme replacement therapy. After three months, they saw improved cardiovascular function in both groups of mice. But only mice that received gene therapy experienced improved breathing function. Improving breathing function is crucial for patients with Pompe disease, who typically rely on ventilators to assist with some or all of their breathing.
In an additional study also presented at the meeting, the researchers performed gene therapy in Pompe mice to determine how the therapy affects the neuromuscular junction.
“Following gene therapy, the Pompe mice began producing the GAA enzyme and crucial functions were restored within the neuromuscular junction,” said Gary Todd, Ph.D., a postdoctoral associate in Falk’s lab. “The success of this correction, however, depended on how advanced the disease was — meaning the earlier in the disease the treatment was given, the better the outcome.”
Researchers now need to conduct additional tests to determine how long the effects of the gene therapy will last, Byrne said.
“In a mouse, the correction is essentially permanent because of how long they live,” he said. “We need to see it in a more representative animal or clinical model to determine the longevity of the therapy.”
Byrne and his team are also working with the biotechnology company Audentes Therapeutics Inc., a company working to develop and commercialize gene therapy products for patients with rare diseases.
“Data from these recent studies provides compelling proof-of-concept data to support clinical gene therapy trials and to bring the therapy to children and adults with Pompe disease,” Falk said.
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic world Gene therapy
Genetic diseases once considered untreatable are now at the center of innovative therapeutic approaches. Research and development of gene therapies in biotech and pharma aim to directly correct or replace defective or missing genes to combat disease at the molecular level. This revolutionary approach promises not only to treat symptoms, but to eliminate the cause of the disease itself.

Topic world Gene therapy
Genetic diseases once considered untreatable are now at the center of innovative therapeutic approaches. Research and development of gene therapies in biotech and pharma aim to directly correct or replace defective or missing genes to combat disease at the molecular level. This revolutionary approach promises not only to treat symptoms, but to eliminate the cause of the disease itself.