FDA Awards Isarna Orphan Drug Designation for ISTH0036 to Improve Glaucoma Treatment Outcome
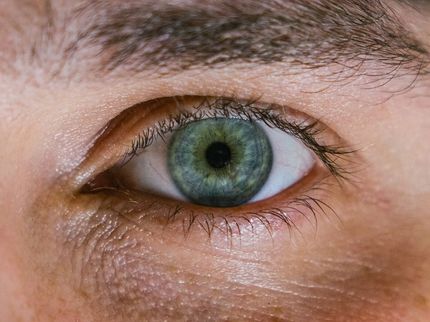
Isarna Therapeutics announced that the Food and Drug Administration (FDA) has granted Orphan Drug Designation for ISTH0036, a locked nucleic acid-modified antisense oligonucleotide, for the prevention of scarring post glaucoma filtration surgery. The FDA decision follows recently announced EMA orphan drug designation for ISTH0036, which is currently in Phase I clinical evaluation in glaucoma patients.
“Both EMA and FDA decisions support the innovation required to improve standard of care for advanced-stage glaucoma patients. Isarna continues to gather insight into the molecular role played by TGF-ß2 in glaucoma pathophysiology and we value the recognition from both agencies of ISTH0036’s potential,” said Dr. Philippe Calais, President and Chief Executive Officer of Isarna Therapeutics.
Glaucoma filtration surgery is the last line of treatment for patients with advanced-stage glaucoma. Although well established as a procedure to lower intraocular pressure and prevent further deterioration of the visual field, scarring counteracts the positive effect of the surgery. ISTH0036 is currently the only compound in clinical development worldwide that directly targets TGF-ß2, a core driver of glaucoma pathophysiology. In addition to glaucoma, several other diseases in ophthalmology have been linked to the modulation of TGF-ß, including diabetic retinopathy, proliferative vitreoretinopathy, and several corneal diseases.
Topics
Organizations
Other news from the department research and development

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.