Stem Cells Might Expand Window for Treating Stroke
A study released today in STEM CELLS Translational Medicine could open the door to a new therapy to potentially expand the current window of time for treating patients after they have experienced a stroke (five times beyond that of a clot-busting drug) and significantly improves their chance of recovery.
Each year, nearly 800,000 people in the United States suffer a stroke. Most of these – nearly 87 percent – are ischemic strokes caused by blood flow to the brain being blocked, usually by a clot. Without oxygen, the brain cells begin to die and death or permanent disability can result. Currently, the only FDA-approved treatment for strokes is tissue plasminogen activator (tPA), a drug that works by dissolving the clot and restoring blood flow. Unfortunately, to be effective, tPA must be administered within about five hours of the stroke and many victims don’t get to the hospital in time to receive it.
Another downside is the drug can exacerbate leakage of the blood-brain barrier (which separates the brain from the circulatory system) and increase the chance for abnormal bleeding. This has left medical researchers searching for something that offers a wider treatment timeframe without negative side effects.
Among the more promising of these is stem cell transplantation, using neural stem cells derived from pluripotent stem cells (iPSC-NSCs) reprogrammed from the patient’s own cells. These cells appear to possess multiple therapeutic benefits, including replacing the damaged brain cells and fighting inflammation.
In the study, researchers from Tulane University conducted studies testing the effectiveness of iPSCs during the early stages of an ischemic stroke. Lead investigator Jean-Pyo Lee, Ph.D., of Tulane’s Center for Stem Cell Research and Regenerative Medicine and Department of Neurology, explained, “A good deal of stroke injury results from reperfusion – when the blood flow is restored after the stroke. If left unchecked, reperfusion can cause an irreversible second opening of the blood-brain barrier within 24 to 72 hours after the first stroke occurs.”
“It is this second episode that contributes significantly to cell death,” she said. “The goal of our study was to find a way to limit early-stage blood-brain barrier injuries, an outcome that would protect against the second phase of stroke damage.”
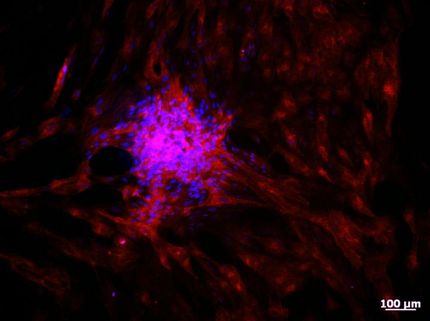
Using mice as their test model, 24 hours after stroke the researchers transplanted iPSC-NSCs into the animals’ ipsilesional hippocampus, a natural niche of NSCs in the brain. In another 24 hours (48 hours after stroke), the stem cells had migrated to the stroke lesion and quickly improved the animals’ neurological function. Recovery of behavioral function persisted throughout the month-long monitoring period, the team reported.
“The rapid therapeutic activity is not due to cell replacement but to a bystander effect that elicits reduced inflammation and blood-brain barrier damage,” Dr. Lee said. “The results have clinical implications by indicating a much extended treatment window (24 hours as opposed to the maximum five hours for tPA). Plus there is the potential for a synergistic effect by combining transplanted hiPSC-NSCs with tPA to reduce a stroke’s adverse effects.”
Original publication
“Bystander effect fuels human induced pluripotent stem cell derived neural stem cells to quickly attenuate early stage neurological deficits after stroke.”; STEM CELLS Translational Medicine
Original publication
“Bystander effect fuels human induced pluripotent stem cell derived neural stem cells to quickly attenuate early stage neurological deficits after stroke.”; STEM CELLS Translational Medicine
Topics
Organizations
Other news from the department science

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.