Scientists Gain Unique Insight into the Function of a Key Muscle Protein
Advertisement
Thanks to the first high-resolution structural analysis of the muscle protein α-actinin, scientists now have a better understanding of how muscles work. The analysis provides crucial information about the structure and function of this complex muscle protein and could lead to the development of new treatments for major muscular disorders. The results of the project, which is funded by the Austrian Science Fund FWF and the European Commission, were recently published in Cell.
Muscles move many things – but first and foremost themselves. Filaments of special proteins pull against each other so that the muscle can exert force. This only works if there is a fixed point, which anchors the filaments. These locations are known as Z-disks and are largely composed of the protein α-actinin. An international research team headed by Kristina Djinovic-Carugo from the Max F. Perutz Laboratories of the University of Vienna and Medical University of Vienna has taken a closer look at this protein.
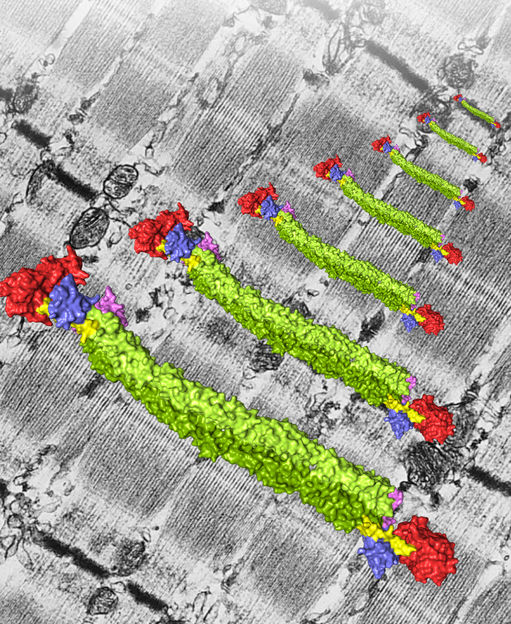
In a muscle every protein has to pull its weight. Thanks to high-resolution structural analysis the role of the essential muscle protein α-Actinin is now better understood.
© Gautel / Ghisleni / Pinotsis / Djinovic-Carugo
Function Follows Form
"We not only succeeded in describing the exact structure of the protein for the first time", explains Djinovic-Carugo, "we were also able to confirm the long-held assumption about how its function is regulated." It emerged from the structural research that showed that α-actinin exists as a dimer, a complex consisting of two identical molecules, and that it displays a cylindrical shape, 360 Å in length and 60 Å wide. Each individual molecule of the dimer has a head-and a neck-like structure followed by a four-part rod-shaped extension.
Two protein domains protruding from the rod-shaped extension in an L-shaped formation proved to be particularly interesting. "These L-shaped domains connect to the neck of the other molecule and this interaction is important for function", describes Djinovic-Carugo. "However, the really exciting discovery about these domains arose when we added the fatty acid molecule PIP2."
Scientists had actually speculated for years that PIP2 plays a key role in the functioning of muscle α-actinin. This hypothesis remained unconfirmed, however, until the following observation was made during the study of Djinovic-Carugo and her international colleagues in Germany, United Kingdom, Norway, Russia, Switzerland and Slovenia: as long as there is no PIP2 available, the L-shaped domain remains connected to the neck of the second α-actinin. If PIP2 is available, the connection opens and renders the domain available to bind to another muscle protein – titin. The trick here – as revealed by the structural data from this FWF project – is that the neck region of the α-actinin is similar in structure to titin. If there is no PIP2, one of the L-shaped domain of an α-actinin molecule binds to a titin-lookalike region in the neck of the opposing molecule. If PIP2 is present, the L-shaped part detaches from the neck and binds titin. The presence of PIP2 is sufficient to change the binding parameters in such a way that the one is prioritised over the other.
X-ray View of The Crystal Ball
Regarding the methodology used in the study, Djinovic-Carugo says: "To deduce the functioning of a protein from its structure, you have to be able to identify everything down to a billionth of a metre. This is only really possible using X-ray diffraction, in which X-ray beams diffract when they encounter the fine structures of a protein, which is presented in the form of a crystal." However, the decision to use this technology involved a tough test of the scientists’ patience at the outset: it took years to produce sufficient amounts of α-actinin to grow the protein crystals. The clarification of how α-actinin is regulated by PIP2 necessitated the use of other complicated complementary analysis methods, and this is where the expertise of Djinovic-Carugo’s international colleagues was indispensable. The comprehensive findings, which were recently acknowledged through the publication of the study in Cell, show that the long and concerted effort was worthwhile.
The importance of the project’s results extends far beyond the basic insights they provide. α-Actinin plays a role in the causes of life-threatening muscular disorders like dystrophies and cardiomyopathies. The new insights into the structure and function of this protein could lead to the development of new approaches to their treatment.

























































