Fruit fly studies shed light on adaptability
Advertisement
An international team of researchers at German Center for neurodegenerative diseases (DZNE) and Tokyo Institute of Technology (Tokyo Tech) have revealed in a collaborative study that neurons change on the molecular level when they are exposed to prolonged light. The researchers could identify that a feedback signalling mechanism is responsible for these changes. The innate neuronal property might be utilized to protect neurons from degeneration or cell death in the future.
Changes in the functional connections between neurons – ‘synapses’ – contribute to our ability to adapt to environmental changes. However until now, little was known about the signalling underlying such ‘synaptic plasticity’. Now, investigations of fruit flies by researchers at the German Center for Neurodegenerative Diseases, Tokyo Tech, the National Institute of Genetics in Japan, and the European Neuroscience Institute in Germany reveal details of the mechanisms behind synaptic plasticity.
Recent studies have suggested that changes in a region at the presynaptic membrane, described as the active zone, control synapse function. The research teams based in Germany and Japan exposed living fruit flies – the commonly studied Drosophila – to different light regimes and then compared the active zones in the photoreceptors.
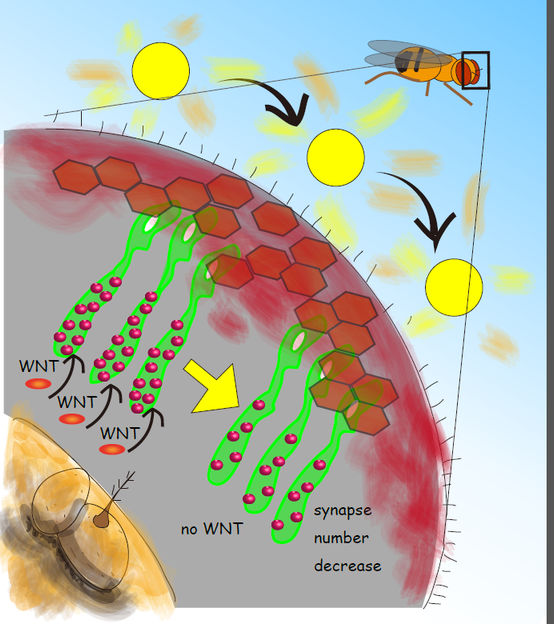
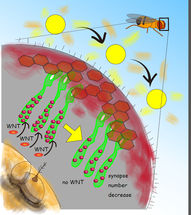
Schematic of the Drosophila visual system showing photoreceptor neurons (green) extending their axons to form synapses (red dots) with the brain neurons. When the fruit fly is exposed to natural light for 3 days the synapse number decreases. This suggests that the neuronal circuit plastically transforms by decreasing the number of efficient connections upon environmental changes, i.e. the light stimuli change. This research showed that the feedback signal mediated by the secreted protein WNT is required for the synapse change.
Takashi Suzuki
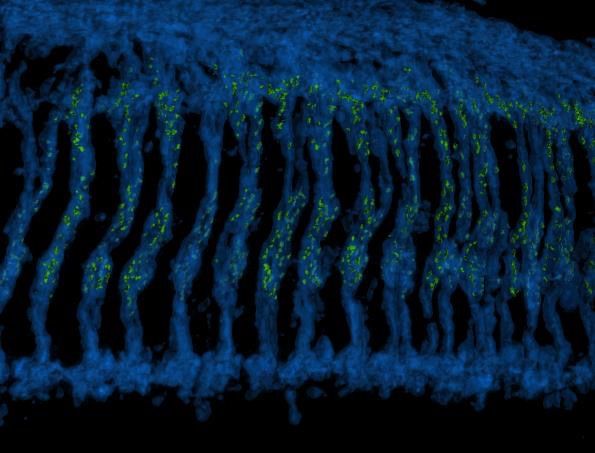
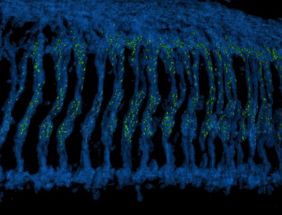
A 3D reconstructed image of the Drosophila optic ganglion where photoreceptor axons (blue) extend downwards to make connections with brain neurons. The synaptic connections formed by photoreceptors are highlighted with the active zone protein Bruchpilot and colored in green.
Atsushi Sugie
T-shaped structures at the presynaptic membrane tether synaptic vesicles and control the release of neurotransmitters to the postsynaptic neuron. By tagging proteins that are crucial to these T-shaped structures the researchers revealed a drop in a subset of active zone proteins, while others remained unchanged. Further, they found that corresponding to the loss of structural proteins, the number of T-shaped structures was also reduced.
The researchers were also able to identify that a feedback mechanism was responsible for these changes and that it relied on the signalling protein Wnt. The results contribute to a better understanding of the molecular mechanisms underlying brain functions such as learning and memory. Future work may investigate how modifying the Wnt signal can be used to manipulate synaptic plasticity, with possible therapeutic applications for neurodegenerative or mental diseases.
“This synaptic change might reflect an innate neuronal property that leads to protection from excessive stimuli,” explains Dr. Atsushi Sugie, the study's lead author. “By enhancing this property, we might be able to protect neurons from degeneration or cell death.”
Original publication
Atsushi Sugie, Satoko Hakeda-Suzuki, Emiko Suzuki, Marion Silies, Mai Shimozono, Christoph Mohl, Takashi Suzuki* and Gaia Tavosanis; "Molecular Remodelling of the Presynaptic Active Zone of Drosophila Photoreceptors via Activity-Dependent Feedback"; Neuron 86, 1-15, 2015.