Biotech materials made simple – crystal structures altered by a single protein
Advertisement
Nacre is not just something pretty to be used for jewellery and decoration. It possesses an intricate layer structure with high strength and hardness, and the naturally formed crystals it contains have some particular properties. This is why industry is working to produce similar materials using biological models. Scientists in Haifa and Saarbrücken have now succeeded in replicating the combination of calcium carbonate and biopolymeric compounds which nature took millions of years and a host of environmental factors to achieve. Using a very simple method, they have been able to show that a single protein species is enough to produce specific effects on the formation of crystal structures.
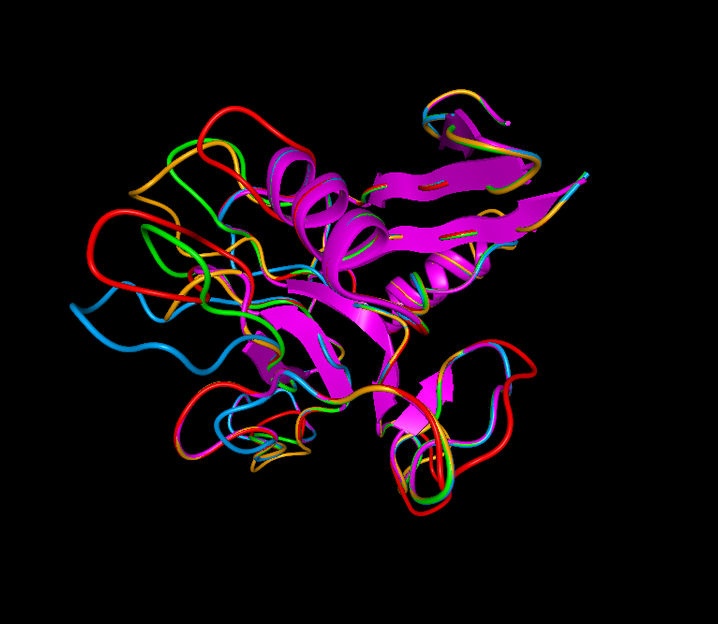
Perlucin has several characteristic protein strands, here indicated by colored loops in a BallView model. They are assumed to cause the observed structural alterations in calcium carbonate crystals.
Copyright INM
The results of their research have recently been published as a cover publication in the journal Chemistry of Materials.
In nacre, layer lattices of inorganic calcium carbonate alternate with layers of organic material. Chitin, collagen and various proteins ensure that the calcium carbonate grows in these defined layers. What role the proteins play during growth had not previously been explained, but the assumption was that several proteins acted together to control the structure of the calcium carbonate lattice as well as themselves forming part of the nacreous layers. However, Ingrid Weiss of the INM – Leibniz Institute for New Materials in Saarbrücken and her colleague Boaz Pokroy at the Technion Israel Institute of Technology have now shown that the crystal lattice of calcium carbonate can be altered using just a single protein species.
“This finding simplifies matters and opens up new possibilities for white biotechnology”, says Weiss, who is Head of the Biomineralization Program at the INM. “Until now, white biotechnology has labored under the idea that mineralization could not be recreated using biological models, because it was assumed that it took a combination of several proteins and a number of factors that were not readily understandable to make biomineralization possible”, she explains. If the natural processes appeared too complicated, they would not be pursued in industrial development.
Pokroy and Weiss have now proved that it need not be that complicated. In their experiments, the researchers extracted the protein perlucin from abalone (Haliotis) shells and combined it with green fluorescent protein (GFP), a trick which enabled them to convert the insoluble perlucin to a water-soluble form. They added this solution at different concentrations to a calcium carbonate solution and examined the crystals produced. The results were compared to crystals produced from a pure calcium carbonate solution and crystals produced from a calcium carbonate solution with GFP.
Only the dissolved perlucin was incorporated in the inorganic carbonate lattice, where it produced notable and wide-ranging distortions to the lattice. The effect follows a principle of “all or nothing”: small quantities of protein are already enough to cause defined lattice distortions. Once the distortion starts, it then reproduces itself continually across the lattice. “GFP alone simply coexists with calcium carbonate – it surrounds the calcium carbonate lattice like a jacket without changing it”, explains the biomineralization expert. As in a shell, it seems to be the perlucin that influences the growth and structure of the crystal lattice.
To explain this phenomenon, the researchers used the INM’s expertise in mussel proteins and the expertise in crystal analysis at the Institute in Haifa. This combination made it possible to observe the reactions of perlucin in the crystal lattice. The scientists are now keen to see whether other proteins have specific effects on the structure and functionality of inorganic crystal lattices.















































