United Kingdom Becomes Only Country to Allow Human Germline Modification
The vote on February 24th in the UK House of Lords means that variations of embryonic genetic modification may soon be used in fertility clinics without any required follow-up of resulting children, despite extensive scientific, ethical, and legal objections heard from around the world. The UK is now the only country in the world to allow human germline modification, genetic changes that will be passed on to future generations.
The Center for Genetics and Society (CGS) joins many others who believe that this is a historic mistake. Human germline modification has long been considered the most objectionable of possible biotechnological developments. Rather than cure anyone, these techniques will turn children into biological experiments and sell wildly exaggerated hope to women already in a challenging position. They will also require the procurement of numerous eggs from healthy young women.
The techniques will combine nuclear DNA of an intended mother with mitochondrial DNA of an anonymous egg provider in an attempt to prevent the maternal transmission of a rare form of mitochondrial disease for a small number of women. Unfortunately, mounting evidence suggests that these biologically extreme processes could introduce the very diseases they are designed to prevent, or cause entirely new developmental problems.
The techniques in question are relatively crude and will not in and of themselves create so-called “designer babies,” as that term is typically understood. However they will result in children with DNA from three different people in every cell of their bodies, which will impact a large range of traits in unknowable ways, and introduce genetic changes that will be passed down to future generations through the female line.
“We hope that, at the very least, UK authorities will follow through on the outstanding recommended safety and efficacy studies prior to any use in humans. They must also ensure that any women considering using these techniques are provided full and objective information about the alternatives available to them for forming healthy families, and about the risks to which they are subjecting their future children,” said CGS Executive Director Marcy Darnovsky, PhD.
This bill enacts an exception to the UK’s law against inheritable genetic modification, which is also prohibited by more than 40 other countries and several international human rights treaties. Despite the gravity of the legal precedent now set by the UK, observers have noted a number of irregularities in the consultations and political process that led up to the vote, from under-representing public and scientific critiques, to using terminology that minimizes the severity and novelty of the manipulations.
“Unlike experimental gene therapies where risks are taken on by consenting individuals, these techniques turn children into our biological experiments and forever alter the human germline in unknowable ways. There is no precedent for this,” Darnovsky said. “We call on those who have supported moving forward with these techniques to make it clear that other kinds of inheritable genetic changes remain off limits.”
Most read news
Other news from the department politics & laws

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents

Loving the sweet enemy - Foods rich in fats and carbohydrates stimulate the reward system in the brain particularly strong

First-in-class immunotherapies for the treatment of cancer and autoimmune diseases - ImmunOs Therapeutics Raises $74 Million Series B Financing Round
BioMed X and Boehringer Ingelheim start new joint research group
Nobilon advances first vaccine into human trials - Intranasal influenza vaccine begins Phase I clinical development
Raptor Pharmaceuticals and TorreyPines Therapeutics Receive Stockholder Approvals to Merge - Merger to Create NASDAQ-Listed Biopharmaceutical Company named Raptor Pharmaceutical Corp.
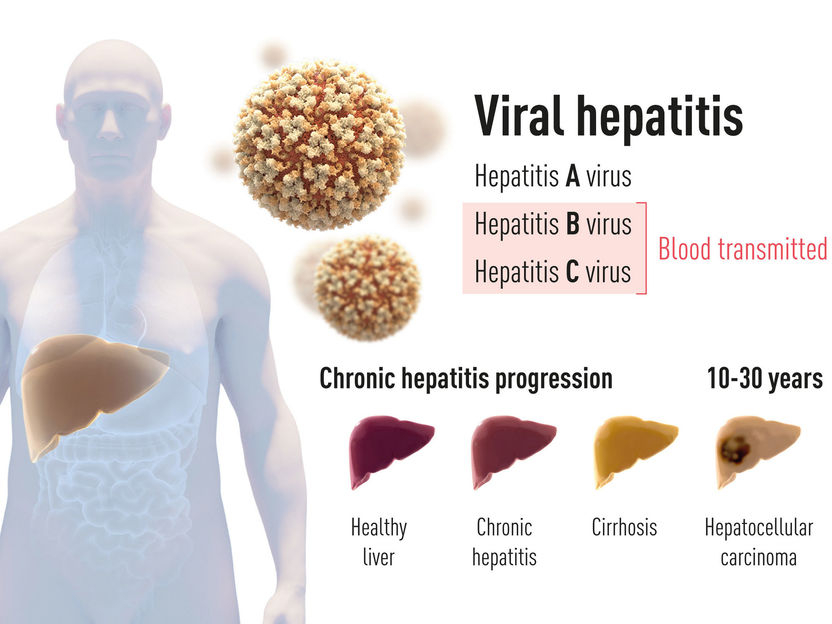
Nobel Prize for Physiology or Medicine 2020 Announced - Nobel Prize awarded to Harvey J. Alter, Michael Houghton and Charles M. Rice for the discovery of Hepatitis C virus
PharmAthene and SIGA Technologies sign definitive merger agreement
PerkinElmer announces third quarter results - GAAP Revenue of $548 million versus $563 million in the comparable prior period

Glox Therapeutics Secures £4.3M Seed Funding to Develop Precision Antimicrobials Targeting Drug-resistant Bacteria - Spin-out from the Universities of Glasgow and Oxford

Doped by food - Dopamine release regulates our eating behaviour

Turning fallen leaves into sustainably made paper - Ukrainian scientist selected as a finalist for the Young Inventors Prize 2024