Stem Cell Therapies Gathering Momentum but Significant Challenges Remain, says GlobalData
Advertisement
With 104 programs in late-stage clinical development, the stem cell therapy space could reach the commercialization threshold by 2017, but a number of challenges remain, according to research and consulting firm GlobalData.
The company’s report "PharmaSphere: Emerging Biotechnologies – Stem Cell Therapy" states that complicated manufacturing processes, untested regulatory pathways and a demanding economic landscape are the largest barriers to progress in the stem cell sector, with a number of companies finding the business unviable.
Aparna Krishnan, MS, GlobalData’s Analyst covering Healthcare Industry Dynamics, says that the financial challenges to the industry have been profound, intensified by the technology’s high failure rates.
Krishnan explains: “Most firms operating in stem cells are undercapitalized and rely heavily on research grants, leveraged finance, and capital raised from public offerings or venture capital investment to fund their research efforts and expansion.
“As a result, the recession impacted the flow of investments into the sector at a crucial time in its evolution. Many start-ups and small companies that form a significant part of the industry lacked financial discipline and often over-leveraged themselves.”
Despite this, GlobalData says that there has been increasing investment capital flowing in from venture capital firms, reaching a maximum of $200 million in 2012, and there are continued signs of investment strength through 2013 and 2014.
The analyst continues: “With the economy stabilizing, attention from major drug firms such as Pfizer and Novartis is growing, as they seek to diversify their businesses in growth segments through licensing deals and acquisitions.
“Also, some stem cell companies, including Bluebird bio and Capricor, have concluded initial public offerings, raising enough capital to continue research and development (R&D) efforts in the process.”
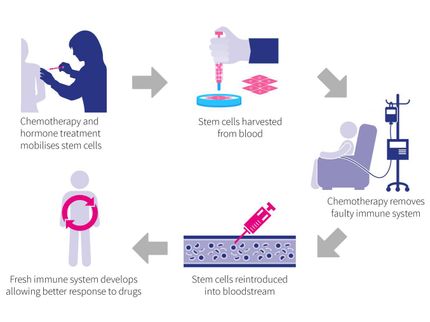
Krishnan adds that the opportunity provided by stem cells in regenerative medicine, among other areas, is clear from the current crop of stem cell companies and their research pipelines.
“The scientific advancement of adult stem cells and the new technology’s demonstrable clinical benefits have led to increasing patient acceptance. As such, GlobalData believes that stem cells are likely to become an integral part of the pharmaceutical industry’s R&D process,” the analyst concludes.