An “anchor” that keeps proteins together
Researchers discover new scaffold protein
All organisms react to different external and internal stimuli: if, for example, the hyphae fungus Sordaria macrospora is supplied with food, it produces fruiting bodies as part of its oestrous cycle. To initiate this reaction, signals have to be transmitted within the cell, which are conveyed by proteins. Physical proximity is a fundamental requirement for different proteins to be able to communicate with each other. Generating that proximity is what scaffolding proteins do, by binding like an anchor to several proteins and keeping them together for the duration of signal transmission. Under the auspices of Dr Ines Teichert, RUB biologists have discovered a new scaffold protein in hyphae fungi. PRO40 is particularly important for the production of fruiting bodies. The researchers report their findings in “PLoS Genetics”.
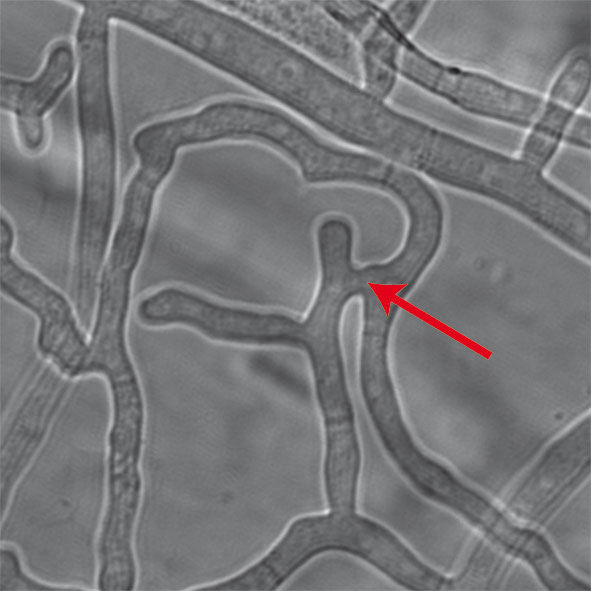
Just like in humans, in fungi, too, fusions between cells are crucial for their development. In the hyphae fungus Sordaria macrospora such fusions (arrow) are required for the production of fruiting bodies during sexual development. The scaffold protein PRO40 is essential for these processes.
© Teichert
Understanding the process is important for fighting diseases
The most important signal pathways of all eukaryotic organisms – that includes humans – are, among others, the so-called MAP kinase modules. By linking phosphate groups, those modules, which are made up of several kinases, cause other proteins to alter their activity. How exactly a small number of MAP kinase modules (in fungi it's mostly three modules) elicit the correct responses to different stimuli, has not, as yet, been fully understood. ”And yet understanding this process is important for fighting diseases which are caused by altered signal transmissions, e.g. Alzheimer’s and cancer,” explains Ines Teichert.
Scaffold proteins make interactions possible
To research into these principles, scientists use organisms with simple life cycles, such as fungi. By using so-called scaffold proteins, detecting the mechanisms underlying signal transmission becomes possible. Like an anchor, they bind different kinases of MAP kinase modules, thus bringing them in close physical proximity with each other. Only then, reactions can take place between them and a signal can be transmitted. Due to scaffold proteins being very dissimilar from each other, they are difficult to find.
No PRO40, no oestrous cycle
Dr Ines Teichert (Department of General and Molecular Botany) and her collaboration partners from the work group for Analytical Chemistry (Dr Dirk Wolters) suspect the protein PRO40 to be a scaffold protein. It has been shown that genetically modified fungi that did not produce PRO40 did not produce any fruiting bodies, either. Examining the proteins, the researchers proved that PRO40 binds two kinases of the MAP kinase module plus an additional kinase that switches on the module. Moreover, PRO40 directly affects the activity of the MAP kinase. “Thus, we were the first ones to demonstrate that PRO40 is a scaffold protein that is specific for sexual development processes in hyphae fungi,” explains Dr Teichert. Scientists know about corresponding substances in other fungi which are responsible for phyto pathogenicity in fungi, i.e. the infestation of plants, or for fungus-plant symbioses. PRO40’s function as a scaffold protein provides, for the first time, a function model on the molecular level.
Financed by the DFG
The German Research Foundation (DFG) has financed the project headed by Prof Dr Ulrich Kück at the Department of General and Molecular Botany (PAK489).
Original publication
Ines Teichert, Eva Katharina Steffens, Nicole Schnaß, Benjamin Fränzel, Christoph Krisp, Dirk A. Wolters & Ulrich Kück (2014) PRO40 is a scaffold protein of the cell wall integrity pathway, linking the MAP kinase module to the upstream activator protein kinase C
Original publication
Ines Teichert, Eva Katharina Steffens, Nicole Schnaß, Benjamin Fränzel, Christoph Krisp, Dirk A. Wolters & Ulrich Kück (2014) PRO40 is a scaffold protein of the cell wall integrity pathway, linking the MAP kinase module to the upstream activator protein kinase C
Organizations
Other news from the department science

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.