500 million year reset for the immune system
Scientists at the Max Planck Institute of Immunobiology and Epigenetics (MPI-IE) in Freiburg re-activated expression of an ancient gene, which is not normally expressed in the mammalian immune system, and found that the animals developed a fish-like thymus. To the researchers surprise, while the mammalian thymus is utilized exclusively for T cell maturation, the reset thymus produced not only T cells, but also served as a maturation site for B cells – a property normally seen only in the thymus of fish. Thus the model could provide an explanation of how the immune system had developed in the course of evolution. The study has been published in Cell Reports.
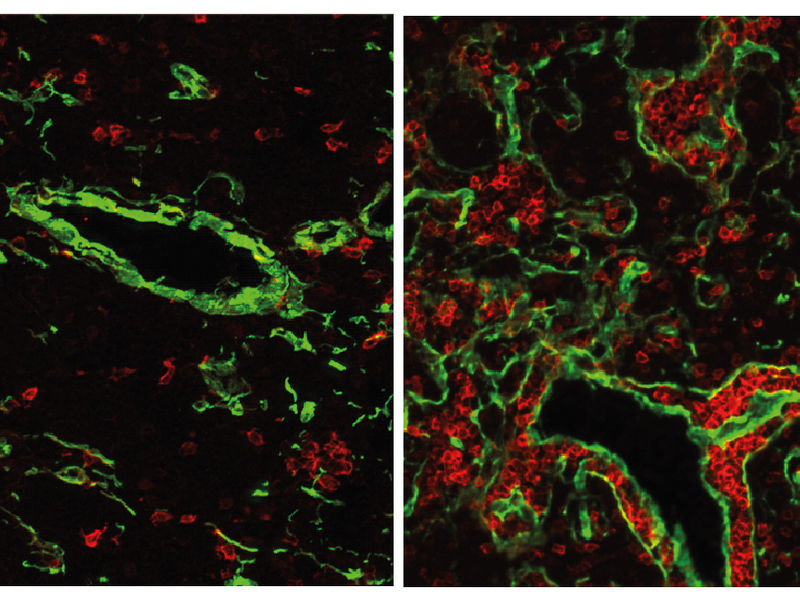
The normal mouse thymus (left) contains only a small fraction of B-cells (red). If the gene FOXN4 is activated, a fish-like thymus with many B-cells develops. This state is likely to have existed about 500 million years ago, at the time when the first vertebrates emerged.
© Max Planck Institute of Immunobiology & Epigenetics
The adaptive immune response is unique to vertebrates. One of its core organs is the thymus, which exists in all vertebrate species. Epithelial cells in the thymus control the maturation of T-cells, which later fight degenerated or infected body cells. The gene FOXN1 is responsible for the development of such T-cells in the mammalian thymus. Scientists led by Thomas Boehm, director at the MPI-IE and head of the department for developmental immunology, activated the evolutionary ancestor of FOXN1, called FOXN4, in the thymic epithelial cells of mice. FOXN4 is present in all vertebrates, but appears to play only a role in the maturation of immune cells of jawed fish, such as cat sharks and zebra fish.
“The simultanuous expression of FOXN4 and FOXN1 in the mouse led to a thymus that showed properties as in fish,” said first author Jeremy Swann. Together with earlier results this suggests that the development and function of thymic tissue was originally intitiated by FOXN4. Due to an evolutionary gene duplication, which led to FOXN1, transiently both genes, and finally only FOXN1 were active in the thymus.
To the researchers surprise not only T-cells developed in the thymus of the mice, but also B-cells. Mature B-cells are responsible for antibody production. In mammals, they normally do not mature in the thymus, but in other organs, such as the bone marrow.
“Our studies suggest a plausible scenario for the transition of a bipotent lymphopoietic tissue to a lymphoid organ supporting primarily T cell development,” said Boehm. Since B- and T-cell progenitors can not yet be distinguished, it remains unclear whether the B-cell development is based on the migration of dedicated B-cell precursors to the thymus, or to maturation from a shared T/B progenitor in the thymus itself. Comparative studies often suggest that the origin of a particular evolutionary innovation must have occurred in an extinct species. „Here, the re-creation and functional analysis of presumed ancestral stages could provide essential insights into the course of such developments," explained Boehm the study approach.
Original publication
Swann JB et al.; Conversion of the thymus into a bi-potent lymphoid organ by replacement of Foxn1 with its paralog Foxn4; Cell Reports, 14 July 2014
Most read news
Original publication
Swann JB et al.; Conversion of the thymus into a bi-potent lymphoid organ by replacement of Foxn1 with its paralog Foxn4; Cell Reports, 14 July 2014
Organizations
Other news from the department science

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents

Spoiled oranges shine light on malignant cells - Biomedical device passes the litmus test

Better infection diagnostics through spin-off at BNITM - (Ex-)employees found start-up "Panadea Diagnostics”
Wolfram_syndrome






















































