A Sweet Pathway into the Cell
Freiburg researchers engineer bacterial proteins that can transport substances across the cell membrane
Advertisement
How do bacteria overcome the barrier of the outer membrane to gain access to the cells of the body? That is the question addressed by junior professor Dr. Winfried Roemer and his colleagues Kevin Troendle and Dr. Julie Claudinon from the Institute of Biology II, members of the Cluster of Excellence BIOSS Centre for Biological Signalling Studies of the University of Freiburg. Roemer uses his findings to introduce drugs into cells over the same path so that they can take effect there. Now he has succeeded in creating a modifiable version of a bacterial protein that could serve in the future as a means of transport into the cell. In addition, the team discovered that the precise geometry of the synthetic bacterial substance plays a role in the recognition of the cellular receptor and the process of absorbing the substance into the cell.
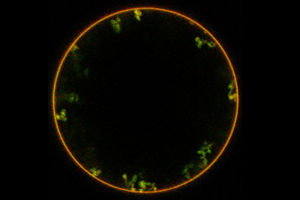
In an experiment on synthetic vesicles (red) the researchers showed that neolectin (green) leeds to invaginations of the membrane. By exploiting this pathway neolectins could be used to transport substances inside cells.
Winfried Römer
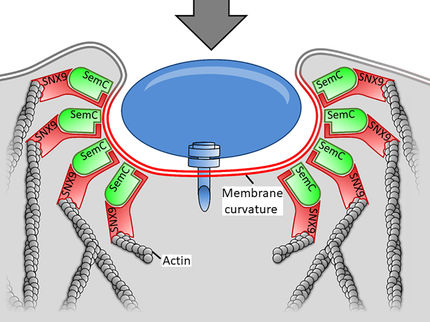
Lectins are proteins that can be formed by bacteria. They bind to sugars distributed on signaling proteins on the surface of the host cell, thus activating a cascade of signals in the cell. The outer membrane then folds in to cover the protein and transport it inside the cell. Bacteria use this process, referred to as endocytosis, to penetrate into the cell and reproduce. Römer has now developed such proteins in cooperation with Dr. Anne Imberty from the University of Grenoble, France. These “neolectins” bind, much like lectins, to the sugar residue in the cell. For the first time the researchers succeeded in deliberately varying the amount of bonds for sugar with the synthetic proteins. “This enables us to design and optimize the neolectins for use as tools for fundamental research, targeted absorption of drugs, and early detection of tumor cells,” explains Römer.
The scientists created the proteins by adding modified lectin genes to bacteria. The microorganisms then produced the neolectins. In this way the scientists engineered 13 proteins whose architecture does not differ from that of the original at first sight. However, the researchers changed the amount of binding sites for sugar and the distance between them. These two factors proved to be critical for the absorption and effect of the neolectins. The scientists first tested the proteins in the lab on surfaces to which sugar was bound. In addition, they studied the neolectins on large synthetic vesicles, bubbles made of fatty acids, also known as liposomes, that were designed to simulate cell membranes. In all of their tests they found neolectins that are especially good at binding. The researchers identified that in order for the cell membrane to fold inward it needs to be at the right proximity to the sugar binding pockets. But the amount of bonds also plays an important role: At least two are necessary for the neolectin to be absorbed quickly into the cell.