The pirate in the microbe
Advertisement
Pirates could have copied the technique they use to capture ships from bacteria. Like buccaneers who draw their boat to a target ship with grappling hooks, the single-cell organisms use threadlike appendages, called pili, to creep along a surface. A research team from the Max Planck Institute of Colloids and Interfaces in Potsdam-Golm and University of Cologne have now developed a model to describe the organisms’ movement. Biologists have long known that microbes attach pili to a substrate and then haul themselves along by reeling them in. However, some microbes, such as Neisseria gonorrhoeae, the pathogen of gonorrhoea, extend their pili in all directions. The path they take thus depends largely on which pilus exerts the strongest pull. The researchers can now explain why bacteria nevertheless are able to travel along a straight line for at least one to two seconds. Detailed knowledge of this mechanism is furthering our understanding of how bacteria infect cells, and could shed light on ways to combat them.
If pirates were to find themselves in a situation comparable to the movement of Neisseria gonorrhoeae, the game would soon be up, because they would quickly be ringed in by naval vessels. And if they then sought the best defence in attack, the situation would spiral into a complete farce. Instead of taking over the fastest ship and escaping on it, the freebooters would engage in a kind of tug-of-war to determine which boat they should draw themselves to with their grappling hooks. That, at least, is more or less how N. gonorrhoeae moves on the surface of a host cell.
To decide which direction to take to form a colony with other bacteria of the same species or to find the best port of entry into a cell, the bacterium engages in a tug-of-war with a random outcome. However, a team of researchers headed by Stefan Klumpp, who leads a Research Group at the Max Planck Institute of Colloids and Interfaces, and Berenike Maier, who conducts research with her team at the University of Cologne, have now found that the microbes do not wander about as randomly as one might expect.
As they explore a surface, the bacteria take somewhat larger steps than would be the case along a purely random path. In this way they are able to explore their surroundings more quickly to find a suitable entry into a host cell or to track down other bacteria to set up a colony.
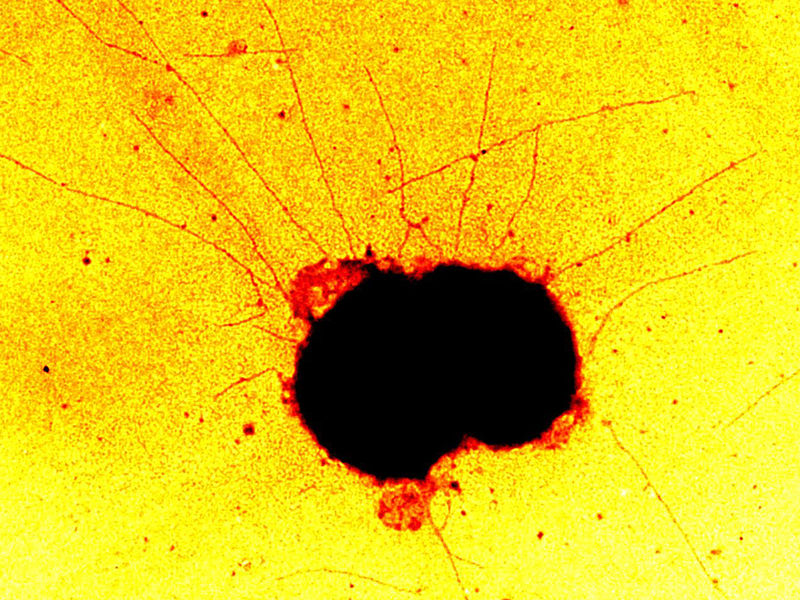
A bacterium faced with a constant decision on which direction to move. Neisseria gonorrhoeae moves across surfaces by extending threadlike appendages, known as pili, in all directions. It attaches the pili to the substrate and then reels them in. It then hauls itself along the pilus that exerts the strongest pull. However, the bacterium’s route is not as random as might be expected from a tug-of-war with a random outcome.
© University of Cologne
The model of one-dimensional tug-of-war cannot be simply expanded
Not only the decisions of some bacteria on which direction to travel end in a tug-of-war. Cells also use this mechanism to determine where to transport enzymes and other biological molecules. The spindle apparatus, along which chromosomes align during cellular division, also engages in a kind of intracellular athletic contest. Within a cell, the tug-of-war usually involves pulling in opposite directions, such as teams have done since antiquity. Biophysicists have a very good understanding of this one-dimensional case. But Neisseria gonorrhoeae is different. “Until now, there has only been a model for a one-dimensional tug-of-war in cells,” says Stefan Klumpp. “If we simply expand this model to two dimensions, the theoretical predictions do not agree with the experimentally observed behaviour of the bacteria.”
In a one-dimensional tug-of-war, the side that gets the upper hand and the direction in which cargo is transported are determined by a random process. Extended to two dimensions, this would mean that N. gonorrhoeae must constantly change its direction once it has extended one of its grappling hooks a certain distance and retracted it again, thus hauling itself forward. “However, in our experiments we observed that the bacterium keeps moving in the same direction for more than one pilus length,” says Berenike Maier, who heads the experimental part of the study at Cologne University. She and her colleagues observed the microbes creeping across protein-coated glass plates. They also observed how the bacteria are able to tug tiny beads out of the focus of laser tweezers with astonishing physical strength.
Mechanical memory of the movement direction
Based on the experimental findings, Stefan Klumpp and his colleagues have developed a computer model that realistically describes the paths the bacteria take. “We’ve discovered two mechanisms that impart directional memory to the bacterium when it is travelling along a straight line,” says the scientist. “When we incorporate these into our model, we find that the results agree very well with the experimentally observed behaviour.”
Together with Alexander Schmidt of the Center for Molecular Biology of Inflammation at the University of Münster (ZMBE), the researchers discovered that the bacterium extends a bundle consisting of two or three pili in the same direction. This increases the likelihood that the microbe will consecutively use multiple pili in the bacterial tug-of-war, thus enabling it to continue moving in the same direction. The odds of this happening are also increased because, having reached a site at which it has just retracted a pilus, the bacterium is immediately able to extend another one in the same direction. This is made possible by a protein complex on the cell wall which continuously assembles bacterial grappling hooks from its constituent elements. After reeling in a pilus, it is able to extend a new pilus in the same direction. “The directional memory of Neisseria gonorrhoeae is therefore based on purely mechanical processes,” says Berenike Maier.
An understanding of microbe’s movement could lead to new therapeutic approaches
The researchers suspect that other bacteria – at least those with a roundish shape that form pili in all directions – increase their step length in a similar fashion. Rod-shaped microbes, by contrast, only extend their motility organs from their two ends and control their path biochemically. However, biochemical signals also play a role in the movement of N. gonorrhoeae: “Biochemical signals probably enable the bacterium to shorten its step length at a potential site of infection, says Stefan Klumpp. In those situations, the microbes rarely form mini-bundles. This helps prevent them from crawling right past a port of entry into the cell.
A detailed understanding of how infectious microbes move with the help of their pili could also bring medical benefits. For example, it could identify possible sites of attack for new antibiotics, because only if the pathogens are able to use their grappling hooks in the usual manner are they able to capture a host cell.