The surprising truth about cell wall growth
Researchers use new techniques to document how cells can conceal growth, then suddenly swell like raisins into grapes; study is a 'paradigm shift' in understanding osmotic shock that may lead to new strategies for fighting bacterial disease.
For a century biologists have thought they understood how the gooey growth that occurs inside cells caused their protective outer walls to expand.
Now, using new microscopic video techniques, Stanford researchers have captured the visual evidence to prove the prevailing wisdom wrong.
"What we observed was not what we had expected," said K.C. Huang, PhD, an assistant professor of bioengineering and the senior author of the findings, which were published online in the Proceedings of the National Academy of Sciences.
The article, which describes a process known as "osmotic shock," was co-authored by Julie Theriot, PhD, a professor of biochemistry and of microbiology and immunology at Stanford's School of Medicine.
The researchers believe their discovery about the surprising resilience of cell wall growth may help explain why seemingly fragile bacteria such as E. coli can thrive in environments as different as puddles and stomachs. Enrique Rojas, PhD, a postdoctoral scholar in bioengineering, and lead author of the PNAS article, is now in Bangladesh trying to apply this knowledge to help fight cholera.
Gurol Suel, PhD, an associate professor of molecular biology at the University of California-San Diego who was not involved in the work, hailed the discovery as "a paradigm shift."
"Just because a hypothesis has been around for decades does not necessarily imply that it is correct," Suel said, adding that the link between internal pressure and cell growth had emerged from less sophisticated experiments, while the Stanford team used modern techniques to "provide a new molecular understanding of bacterial cell growth."
Life under pressure
Cells are the basic structural units of all life. They were first observed more than 400 years ago after the invention of the microscope. One of the most studied cells in science is E. coli, a sausage-shaped bacterium that can cause food poisoning.
In fact, cells resemble sausages insofar as both consist of outer envelopes stuffed with an inner mass. For decades biologists have believed that the growth of this inner mass, pressing on the outer membrane, is what caused cell walls to grow.
However, using new techniques to isolate and visualize cells in different environments, the Stanford team proved that cell wall growth occurred regardless of the pressures exerted on the cell – whether from inside or out.
Here it is critical to understand that, unlike a sausage, the outer envelope of a cell is alive, dynamic and porous. It is designed to allow water to seep in or out. This is important because cells live in fluids and hence are subject to the pressure of osmosis.
Osmosis relates to the amount of solid materials dissolved in a liquid solution. Stirring sugar into coffee, for instance, increases its osmotic pressure. The more sugar you stir in, the higher the osmotic pressure of the solution.
Life is based on water, so cells have an internal osmotic pressure. When a cell enters a solution with a higher osmotic pressure – such as a sugary liquid – its porous membrane tries to protect the cell by letting water out. This causes the cell membrane to shrivel up, compacting the cell to withstand the pressure from without. Put the same cell back into a normal solution, and the porous cell wall allows water to seep back in, causing the cell to swell to its former size.
Biologists have long supposed that this same pressure dynamic retarded cell wall growth. It made sense given the prevailing wisdom – if cell wall growth were indeed driven by expansion from inside the cell, and outward pressure forced the cell to contract, how could the outer cell wall continue to grow?
In fact, the Stanford team initially designed its experiment to measure precisely how much osmotic pressure slowed cell wall growth in E. coli.
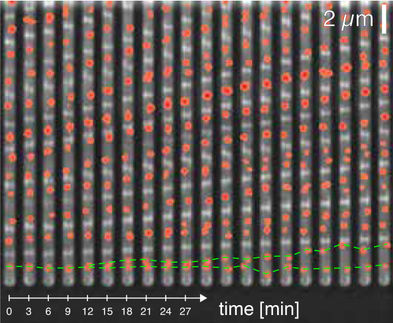
They used microfluidic devices to trap the bacterial cells in tiny chambers. This allowed them to bathe the confined cells, first in highly concentrated sugars (high osmotic pressure), then in normal solutions (low osmotic pressure), while recording precise images of cell contraction or expansion.
Initially, the results seemed to confirm the prevailing wisdom: cells bathed in a sugar solution appeared to grow more slowly.
But whenever the researchers "shocked" the cells by flushing out the sugars and bathing the cells in normal solution they were surprised to see that the cells expanded rapidly – in a matter of seconds -- to a size roughly equivalent to cells growing at full speed in normal solutions. They captured this expansion on video.
"The cells just didn't seem to care that they had been subjected to frequent and large (osmotic) insults in the chamber," Huang said.
The Stanford researchers came to realize that the cell walls had continued to grow in the sugar solution just as fast as in the normal solution – but the extra mass was shriveled like a raisin. When the cell re-entered the normal solution and water seeped back in through the porous membrane, the now-turgid cell smoothed out like a grape, and all the non-apparent growth became visible.
To follow up this surprising finding, Rojas is in Bangladesh, extending the investigations to study how bacterial pathogens such as Vibrio cholerae respond to rapidly changing fluid environments and how to use this knowledge to fight this scourge.