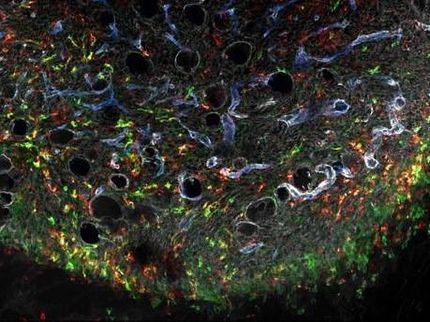
Two-lock box delivers cancer therapy
Rice University scientists have designed a tunable virus that works like a safe deposit box. It takes two keys to open it and release its therapeutic cargo.
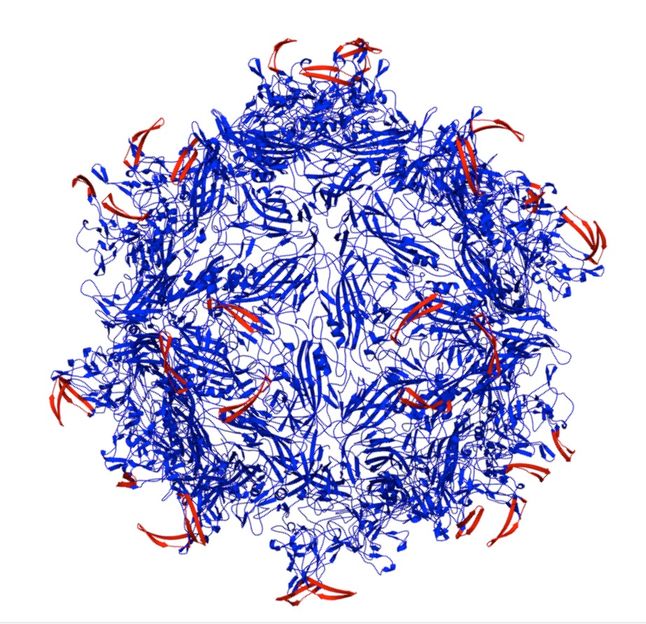
An adeno-associated virus capsid (blue) modified by peptides (red) inserted to lock the virus is the result of research at Rice University into a new way to target cancerous and other diseased cells. The peptides are keyed to proteases overexpressed at the site of diseased tissues; they unlock the capsid and allow it to deliver its therapeutic cargo.
Junghae Suh/Rice University
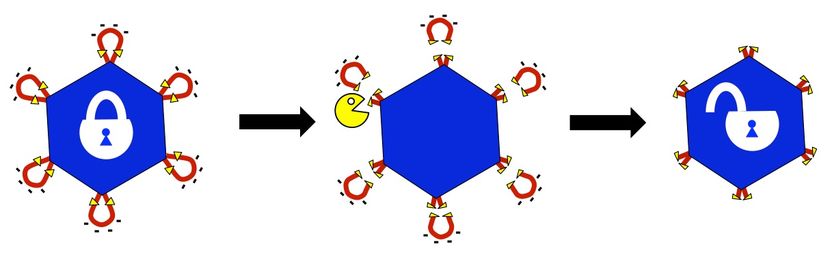
Modified adeno-associated viruses developed at Rice University are “locked” by peptides that respond to the presence of two proteases likely to be overexpressed at the site of cancerous or other diseased tissues. The proteases chew off the peptides, thereby unlocking the virus and allowing it to deliver its therapeutic cargo.
Junghae Suh/Rice University
The Rice lab of bioengineer Junghae Suh has developed an adeno-associated virus (AAV) that unlocks only in the presence of two selected proteases, enzymes that cut up other proteins for disposal. Because certain proteases are elevated at tumor sites, the viruses can be designed to target and destroy the cancer cells.
The work appears online in the American Chemical Society journal ACS Nano.
AAVs are fairly benign and have become the object of intense study as delivery vehicles for gene therapies. Researchers often try to target AAVs to cellular receptors that may be slightly overexpressed on diseased cells.
The Rice lab takes a different approach. “We were looking for other types of biomarkers beyond cellular receptors present at disease sites,” Suh said. “In breast cancer, for example, it’s known the tumor cells oversecrete extracellular proteases, but perhaps more important are the infiltrating immune cells that migrate into the tumor microenvironment and start dumping out a whole bunch of proteases as well.
“So that’s what we’re going after to do targeted delivery. Our basic idea is to create viruses that, in the locked configuration, can’t do anything. They’re inert,” she said. When programmed AAVs encounter the right protease keys at sites of disease, “these viruses unlock, bind to the cells and deliver payloads that will either kill the cells for cancer therapy or deliver genes that can fix them for other disease applications.”
Suh’s lab genetically inserts peptides into the self-assembling AAVs to lock the capsids, the hard shells that protect genes contained within. The target proteases recognize the peptides “and chew off the locks,” effectively unlocking the virus and allowing it to bind to the diseased cells.
“If we were just looking for one protease, it might be at the cancer site, but it could also be somewhere else in your body where you have inflammation. This could lead to undesirable side effects,” she said. “By requiring two different proteases – let’s say protease A and protease B – to open the locked virus, we may achieve higher delivery specificity since the chance of having both proteases elevated at a site becomes smaller.”
In the future, molecular-imaging approaches will be used to detect both the identity and concentration of elevated proteases. “With that information, we would be able to pick a virus device from our panel of engineered variants that has the right properties to target that disease site. That’s where we want to go,” she said.
Suh said elevated proteases are found around many diseased tissues. She suggested these protease-activatable viruses may be useful for the treatment of not only cancers but also neurological diseases, such as stroke, Parkinson’s and Alzheimer’s diseases, and heart diseases, including myocardial infarction and congestive heart failure.
The ultimate vision of this technology is to design viruses that can carry out a combination of steps for targeting. “To increase the specificity of virus unlocking, you can imagine creating viruses that require many more keys to open,” she said. “For example, you may need both proteases A and B as well as a cellular receptor to unlock the virus. The work reported here is a good first step toward this goal.”