Simple microfluidic devices now have valves
Researchers at the National Institute of Standards and Technology (NIST) have added yet another innovation—miniature valves—to their ever-growing collection of inexpensive, easy-to-manufacture and highly efficient microfluidic devices made from plastic films and double-sided tape.
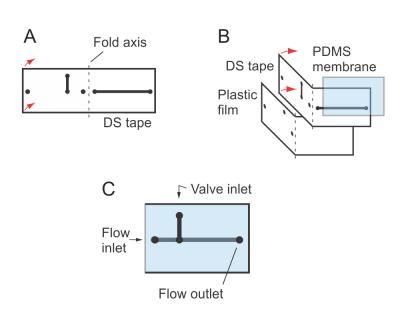
Double-sided tape is cut with channels and ports that will align when folded (A). The polymer membrane that supplies the valve function for the microfluidic device is sandwiched between (B). The completed apparatus (C) has ports for fluid flow into and out of the device, as well as a valve inlet for air. Air pressure pushes the membrane into the flow channel, blocking fluid movement.
Gregory A. Cooksey/National Institute of Standards and Technology
Traditionally, microfluidic devices—tiny gadgets with fluid-carrying channels used in medical diagnostics, DNA forensics and "lab-on-a-chip" chemical analyzers—have been fabricated like microchips using photolithography. A desired pattern of micrometer-sized channels and ports is created on top of a silicon substrate, which can then be replicated many times by techniques such as molding or embossing. However, the process requires specialized cleanroom equipment and can take several days to complete.
If valves are needed in the system, they traditionally have been made from silicones. Unfortunately, silicones are not the best materials to use with particular laboratory assays or for manufacturing lab-on-a-chip structures.
NIST researchers have spent the past few years developing and refining a method for making microfluidic devices using plastic films and double-sided tape that produces a functional apparatus in hours rather than days and requires only simple tools to create channels and ports. The NIST designs allow for folding the films to make multilayer or three-dimensional structures, can be used to make devices with multiple functions, and cost a fraction of traditional fabrication techniques.
But until now, there has not been a practical way to incorporate valves for dynamic control of fluid flow in these devices. In a new paper in the journal Lab on a Chip, NIST bioengineer Gregory Cooksey and research engineer Javier Atencia describe the first-ever technique for building pneumatic microvalves into 2-D and 3-D microfluidic devices made with plastic films and tape.
Like previous NIST systems, the new valved microfluidic device is built in layers. Narrow slits and holes are cut into pieces of double-sided tape that become tiny channels and ports when the tape is folded on itself. The microvalve is made by sandwiching a flexible membrane between two channels that intersect, one on top of the other. Applying air pressure to the top channel pushes the membrane down like a diaphragm valve, closing the lower channel.
Cooksey and Atencia have demonstrated that their novel microvalve also can work with more complex configurations of the NIST microfluidic system. These include devices with different designs for performing different tasks simultaneously, multiple layers with different flow rates, and single units with multiple "microfluidic walls" that can fold together to form a 3-D shape. In one trial with a cubed-shaped device, the researchers filled it with agar and grew nematodes (Caenorhabditis elegans) inside. Using the microchannels, ports and valves built into the cube's walls, they injected chemicals at controlled concentrations that either attracted or repelled the worms. This showed that the cube was a unique setup for studying a living organism's response to chemical stimuli within a closed environment.
Most read news
Organizations
Other news from the department science

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.




















































