Human diseases caused by defective ribosomes
ribosomes are essential for life, generating all of the proteins required for cells to grow. Mutations in some of the proteins that make ribosomes cause disorders characterized by bone marrow failure and anemia early in life, followed by elevated cancer risk in middle age. These disorders are generally called "ribosomopathies."
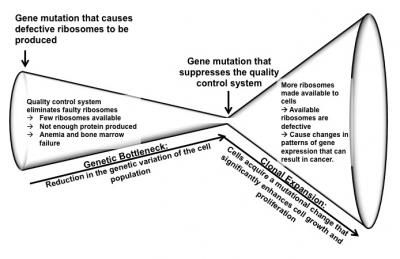
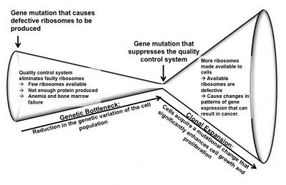
This is a model of ribosomopathy disease progression from anemia to cancer. A ribosomal mutation causes defective ribosomes to be produced. Due to the built-in quality control process, the majority of defective ribosomes carrying the mutation do not pass inspection. This leaves few ribosomes available for cells to use to produce required proteins, which causes anemia and bone marrow failure. A second gene mutation suppresses the quality control system, making more ribosomes available to cells. However, the available ribosomes are defective and cause changes in gene expression patterns that can result in on the onset of cancers, such as T-cell acute lymphoblastic leukemia.
Jonathan Dinman
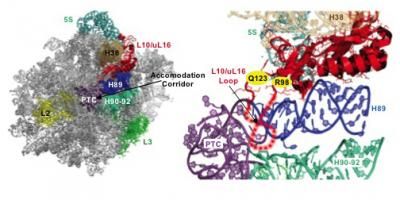
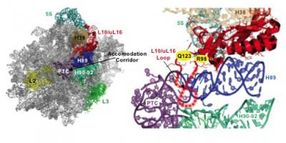
This is the molecular basis for the T-cell acute lymphoblastic leukemia (T-ALL) ribosomopathy. Left: "Crown view" of the large subunit of the ribosome highlighting critical functional parts. tRNAs pass through the accommodation corridor when traveling from outside the ribosome into the catalytic core (PTC). Right: Close-up view of ribosomal protein L10/uL16 and its surroundings. The mutations found in T-ALL patients are shown in yellow. These lie at the base of a loop (dotted lines) that protrudes into the accommodation corridor. When a tRNA passes through the accommodation corridor, it flips the loop up. This functions like a switch or a sensor, signaling to other regions of the ribosome that a tRNA has just passed through. In response, the ribosome undergoes a change in shape that allows it to proceed to the next step in the protein synthetic process. Mutations in the loop displace it, making it less able to do its job.
Jonathan Dinman
How can ribosomopathies first appear as diseases caused by too few cells, but later turn into diseases caused by too many cells? This paradox has puzzled the scientific community for years. A new study, which uses a genetic approach to examine this paradox, suggests ribosomopathies are caused by a sequence of mistakes at the molecular level.
The study proposes a detailed version of this basic chain of events: Some people are born with or acquire a gene mutation that causes defective ribosomes to be produced. A quality control system in cells eliminates most of the faulty ribosomes. This leaves few ribosomes available for cells to use to produce required proteins, which causes anemia and bone marrow failure early in life. Next, a second gene mutation suppresses the quality control system, making more ribosomes available to cells. However, the available ribosomes are defective and cause changes in gene expression patterns that can result in cancer.
The study will be published in the online early edition of the journal Proceedings of the National Academy of Sciences. The research was partly supported by the National Institutes of Health.
"Making ribosomes is a lot like making cars—there is an intricate cellular assembly line where many different parts are brought together to make a complex, fine-tuned, high-performance machine. The assembly line contains quality control inspectors located at critical points in the process to ensure that machines with defective parts do not make it out of the factory and onto the roads," said Jonathan Dinman, professor in the Department of Cell Biology and Molecular Genetics at the University of Maryland. "Imagine a scenario where the only supplier of a specific part produces a defective one. If the inspectors do their jobs, very few new cars will reach the market. This scenario would put most car companies out of business—this is equivalent to bone marrow failure. In the meantime, the demand for new cars increases. This opens the door for an unscrupulous company to fire their inspectors and flood the market with 'lemons.' While good for the company's bottom line in the short term, in the long term the increased rates of accidents and lawsuits wreak havoc."
Dinman collaborated on this study with Kim De Keersmaecker of the University of Leuven in Belgium; Arlen Johnson of the University of Texas at Austin; and Sergey Sulima, who conducted this research as a graduate student in the Department of Cell Biology and Molecular Genetics at UMD and is now a postdoctoral researcher in De Keersmaecker's lab.
The researchers set out to investigate structural, biochemical and other defects in ribosomes that may lead to cancer. The team selected budding yeast as their model system, as assembly of its ribosomes shares many characteristics with human cells, making it a powerful experimental tool. They used a strain of yeast containing a specific ribosomal protein mutation previously identified in patients with a potentially fast-moving form of leukemia known as T-cell acute lymphoblastic leukemia (T-ALL).
When the researchers grew the mutant yeast cells on a petri dish, the cells grew very slowly. The researchers suggest that because of the cells' built-in quality control process, the majority of defective ribosomes carrying the T-ALL mutation do not pass the inspection. This severely limits the supply of ribosomes available to produce proteins, only providing enough ribosomes for cells to barely survive. This supply-and-demand problem hits rapidly dividing cells like blood cells particularly hard, causing anemia and bone marrow failure early in life. Although these disorders can be medically managed with frequent blood transfusions, the bone marrow cells are subjected to an evolutionary phenomenon called "selective pressure," a process that favors reproduction of individuals that resolve problems that limit their ability to thrive. In this case, cells would be favored that could circumvent the mutation that limits the number of available ribosomes and cellular reproduction.
After a few weeks, a group of fast-growing cells appeared on the petri dish containing the mutant yeast cells. The team sequenced the genomes of these cells and found a mutation in a second gene that codes for one of the quality control inspectors. The mutation made the quality control inspector do its job less accurately. This increased the total number of ribosomes available to the cells, enabling cells with the mutation to make more protein, grow quickly, and take over the population. However, the available ribosomes were still defective: their underlying structural problems and biochemical defects never got repaired.
The researchers found that the defective ribosomes tend to make a specific kind of mistake when translating the genetic code. This mistake changes specific patterns of gene expression in cells, consistent with changes that can lead to cancer. The mistakes make an already unstable set of molecules even more unstable. One such set of molecules is important for helping cells maintain telomeres—the DNA at the ends of the chromosomes. The mutant cells exhibited shortened telomeres, a fundamental defect that has been linked to both cancer and aging. The research team proposed two different, but not mutually exclusive, explanations for the changes in gene expression: the mutant ribosomes could be directly changing patterns of gene expression and/or the second suppressor mutation could be driving the changes.
Currently, the researchers are looking for suppressors in human cells that may promote cancer.
"Our yeast work has established a new paradigm that we are now translating to humans," said Dinman. "Once we determine which ribosomal mutations suppress the quality control system in humans, we may be able to identify a potential drug target."