Understanding PP1, the ubiquitous enzyme
In the Proceedings of the National Academy of Sciences, a team of scientists at Brown University reports a major step forward in determining the specific behavior of the ubiquitous enzyme PP1 implicated in a wide range of diseases including cancer.
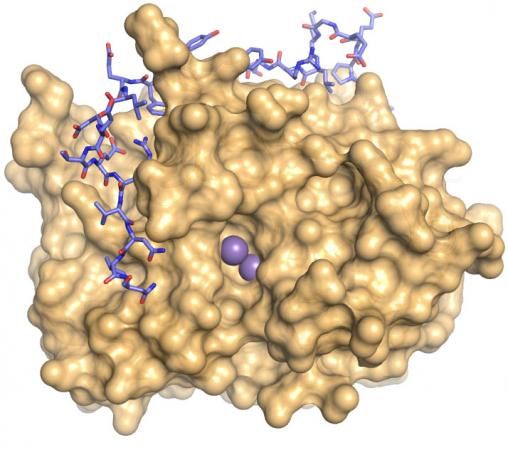
The enzyme PP1, the tan mass above, is everywhere in the body and has a role in nearly every biological process. That role is shaped more than 200 regulatory proteins that bind to PP1, including one called PNUTS, blue and pink above.
Page lab/Brown University
PP1, whose role is to enable the passage of molecular messages among cells, is found pretty much everywhere in the body. Its wide range of responsibilities means it is essential to many healthy functions and, when things go wrong, to diseases. But its very versatility has prevented it from being a target for drug development, said Rebecca Page, associate professor of biology at Brown and the paper’s corresponding author.
“The amazing thing about PP1 is that no one has wanted to touch it for the most part as a drug target because PP1 is involved in nearly every biological process,” Page said. “It’s not like you could just target the PP1 active site for, let’s say, diabetes because then you are going to affect drug addiction, Alzheimer’s disease and all these other diseases at the same time.”
In other words, make a medicine to block PP1 in one bodily context and you’d ruin it in all other contexts. Structural biologists such as Page and Brown co-author Wolfgang Peti have therefore been eager to learn what makes PP1 behave in specific ways in specific situations.
The key is the way PP1 binds with more than 200 different regulatory proteins. Scientists know of these proteins and know the sequences of amino acids that compose them, but they don’t know their structure or how they actually guide PP1.
“The ability to predict how these PP1 interacting proteins bind PP1 from sequence alone is still missing,” Page and her colleagues wrote in PNAS.
Now, through experiments in which her team including lead author Meng Choy combined NMR spectroscopy, X-ray crystallography and techniques in biochemistry, she has learned how PP1 binds to a targeting protein called PNUTS, forming “binding motifs.” That knowledge, combined with what she learned in earlier studies about two other targeting proteins — NIPP1 and spinophilin — has allowed her team to predict how PP1 binds with 43 of the 200 regulatory proteins that give it specific behavior.
“What this work in conjunction with two of our previous structures allowed us to do was to define two entirely new motifs,” she said. “From that, comparing the sequences with the known proteins that interact with PP1 whose structures we don’t have, we were able to predict that 20 percent of them likely interact in a way that is similar to these three proteins.”
So by resolving the structure of just three proteins with PP1, Page now has the means to understand the binding of many proteins without having to resolve their structure. Instead she need only know the few motifs and the proteins’ sequences.
As for PP1’s interactions with the other 80 percent or so of regulatory proteins, those remain a mystery. But Page said the success her team has had in the lab working with PP1 and resolving key motifs makes her optimistic that those interactions can be solved, too.
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic World Spectroscopy
Investigation with spectroscopy gives us unique insights into the composition and structure of materials. From UV-Vis spectroscopy to infrared and Raman spectroscopy to fluorescence and atomic absorption spectroscopy, spectroscopy offers us a wide range of analytical techniques to precisely characterize substances. Immerse yourself in the fascinating world of spectroscopy!

Topic World Spectroscopy
Investigation with spectroscopy gives us unique insights into the composition and structure of materials. From UV-Vis spectroscopy to infrared and Raman spectroscopy to fluorescence and atomic absorption spectroscopy, spectroscopy offers us a wide range of analytical techniques to precisely characterize substances. Immerse yourself in the fascinating world of spectroscopy!