Dangerous mistaken identity
Chaperone binds protein responsible for Alzheimer’s disease
Advertisement
tau proteins, which are responsible for Alzheimer’s disease, bind to the folding protein Hsp90. The molecular recognition mechanisms that play a role here, have been unveiled by an international team of scientists led by the Technische Universitaet Muenchen (TUM) and the Helmholtz Zentrum Muenchen. This might open the door for new approaches for the treatment of Alzheimer’s disease, as the scientists report in the trade journal “Cell”.
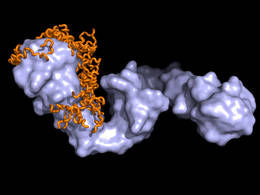
NMR/SAXS structural model of the Hsp90-Tau protein complex (light blue: Hsp90, orange: tau-protein)
Tobias Madl / TUM/HMGU
Proteins like the so-called heat shock protein Hsp90 play an important role in almost all processes within human cells. They help other proteins fold into their three-dimensional structure or return damaged proteins back into their proper shape.
Recently, there has been increasing evidence indicating that the heat shock protein Hsp90 may also be involved in the folding processes of the tau protein. Deposits of tau proteins in brain cells are typical for Alzheimer’s disease and are held responsible for decaying nerve cells.
However, while dissolved tau proteins look more like long, stretched chains, HSP90 binds predominantly proteins that have already been prefolded. This contradiction has now been resolved by an international team headed by Dr. Tobias Madl, leader of the BioSysNet Working Group and TUM Junior Fellow at the Technische Universitaet Muenchen and leader of the Emmy-Noether Group Structural Biology of Signal Transduction at the Institute of Structural Biology at the Helmholz Zentrum München, as well as Prof. Stefan Rüdiger from the Dutch University of Utrecht.
Using a combination of very different technics like magnetic resonance spectroscopy, small-angle X-ray scattering and computer modeling, they successfully determined structure and dynamics of the interactions between the two biomolecules: For Hsp90 the tau protein looks like a prefolded larger protein. Furthermore they were able to deduce how Hsp90 influences the aggregation of tau proteins with one another.
“Deposits of tau proteins can cause Alzheimer’s disease. We have discovered the protein regions in which the proteins interact. This is a novel and important starting point for influencing structural formation and for developing future therapies for Alzheimer’s disease,” says Madl.
In addition to Alzheimer’s disease, further neurodegenerative diseases are caused by protein aggregation. Chaperones also play a role in the development of cancer and cystic fibrosis. These scientific insights thus provide an important basis for better understanding the disease mechanisms.



























































