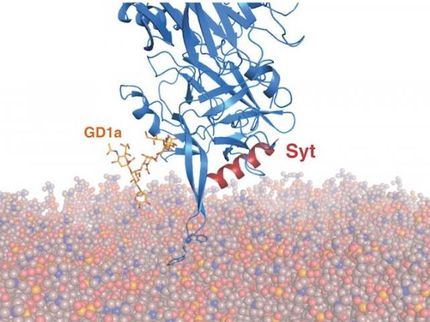
How botox binds to neurons
Researchers elucidate how botulinum neurotoxin A binds to its protein receptor and thus provide a basis for the development of new drugs
Advertisement
Botulinum neurotoxin A, better known as Botox, is a highly dangerous toxin that causes paralysis in man that may prove fatal. In cosmetic applications the paralysing action of small doses is used in a specific manner for the temporary elimination of wrinkles and in medicine as a treatment for migraine or to correct strabismus. An international research team from the Paul Scherrer Institute, Utrecht University and the pharmaceutical company UCB has now taken an important step towards understanding the action of botulinum neurotoxin A. They have determined the x-ray crystal structure of a protein complex which clearly shows how the toxin molecule binds to the protein receptor, synaptic vesicle protein 2. The findings may prove useful for the development of improved botox drugs with a lower risk of overdosage. The structure was determined at the Swiss Light Source synchrotron at the Paul Scherrer Institute.
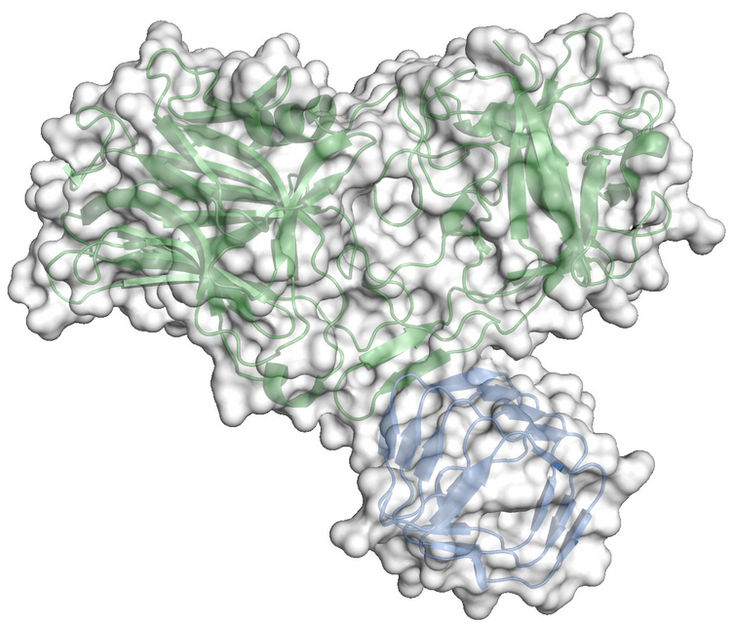
The depicted structure shows how botox binds to the protein receptor synaptic vesicle protein 2 of the neuron. What can be seen is the crystal structure of the complex consisting of the luminal domain of synaptic vesicle protein 2 (blue) and the receptor binding domain of botulinum neurotoxin A (green).
PSI
The consumption of spoiled tinned food can lead to botulism in man, an intoxication that causes life-threatening paralysis. One of the causative factors is the toxin botulinum neurotoxin A which is produced by the bacterium Clostridium botulinum. It can only replicate in the oxygen-free atmosphere of the tin. The toxin attacks the neurons and prevents the passing on of neuronal signals to the muscles. In recent decades, increasingly practical applications of the toxin have been developed. Its use in cosmetics where the substance is called botox is particularly well known. When injected subcutaneously the toxin leads to relaxation of muscles and makes wrinkles disappear temporarily. This agent is also frequently used in medicine to treat migraine. In people suffering from strabismus, botulinum neurotoxin A can be used specifically to slightly weaken the eye muscle and facilitate normal vision.
Synchrotron rays reveal the protein complex structure
One fundamental step, needed to trigger the action of the botulinum toxin, is the binding of a toxin molecule to a molecule of the protein receptor synaptic vesicle protein 2 of the nerve cell. The interaction between the receptor and botulinum neurotoxin A molecule leads to a cascade of events which prevent the neuron from releasing messenger substances that normally encourage muscle movement. An international team headed by Richard Kammerer at the Laboratory of Biomolecular Research at the Paul Scherrer Institute has now succeeded in determining the exact details of the molecular interaction between botox and its receptor. “Our findings are an important step towards understanding the mode of action of botulinum neurotoxin A. I am, therefore, confident that our structure will evoke major interest in the field”, explains Kammerer. For the determination of the structure the researchers used the protein crystallography method. This involves the production of large amounts of the molecules and their arrangement in a regular structure, a crystal. This crystal is then irradiated with x-rays from the Swiss Light Source synchrotron. The principle behind this technology is that x-rays are diffracted by the molecules in the crystal. From these diffraction patterns the researchers can then determine the atomic structure of the molecule under investigation.
New drugs possible
The findings not only help us to better understand the action of botox but may also be of considerable practical benefit. “As a drug, botulinum neurotoxin A has a very narrow therapeutic window”, explains Roger Benoit, researcher at PSI and the first author of the article. “This means that even a minor overdose can have a damaging effect”. With our findings it should be possible to develop drugs with weaker action where the risk of overdosage would then be lower.”