Stem cells to generate myelin
Findings hold promise for developing regenerative therapies for spinal cord injuries and diseases such as multiple sclerosis
Advertisement
Stem cell technology has long offered the hope of regenerating tissue to repair broken or damaged neural tissue. Findings from a team of UC Davis investigators have brought this dream a step closer by developing a method to generate functioning brain cells that produce myelin — a fatty, insulating sheath essential to normal neural conduction.
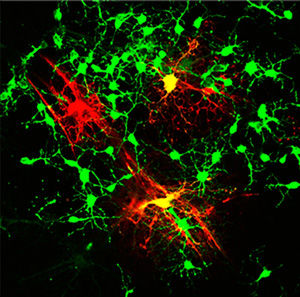
Myelination of spiking and non-spiking mESC-OPCs
© UC Regents
“Our findings represent an important conceptual advance in stem cell research,” said Wenbin Deng, principal investigator of the study and associate professor at the UC Davis Department of Biochemistry and Molecular Medicine. “We have bioengineered the first generation of myelin-producing cells with superior regenerative capacity.”
The brain is made up predominantly of two cell types: neurons and glial cells. Neurons are regarded as responsible for thought and sensation. Glial cells surround, support and communicate with neurons, helping neurons process and transmit information using electrical and chemical signals. One type of glial cell — the oligodendrocyte — produces a sheath called myelin that provides support and insulation to neurons. Myelin, which has been compared to insulation around electrical wires that helps to prevent short circuits, is essential for normal neural conduction and brain function; well-recognized conditions involving defective myelin development or myelin loss include multiple sclerosis and leukodystrophies.
In this study, the UC Davis team first developed a novel protocol to efficiently induce embryonic stem cells (ESCs) to differentiate into oligodendroglial progenitor cells (OPCs), early cells that normally develop into oligodendrocytes. Although this has been successfully done by other researchers, the UC Davis method results in a purer population of OPCs, according to Deng, with fewer other cell types arising from their technique.
They next compared electrophysiological properties of the derived OPCs to naturally occurring OPCs. They found that unlike natural OPCs, the ESC-derived OPCs lacked sodium ion channels in their cell membranes, making them unable to generate spikes when electrically stimulated. Using a technique called viral transduction, they then introduced DNA that codes for sodium channels into the ESC-derived OPCs. These OPCs then expressed ion channels in their cells and developed the ability to generate spikes.
According to Deng, this is the first time that scientists have successfully generated OPCs with so-called spiking properties. This achievement allowed them to compare the capabilities of spiking cells to non-spiking cells.
In cell culture, they found that only spiking OPCs received electrical input from neurons, and they showed superior capability to mature into oligodendrocytes.
They also transplanted spiking and non-spiking OPCs into the spinal cord and brains of mice that are genetically unable to produce myelin. Both types of OPCs had the capability to mature into oligo-dendrocytes and produce myelin, but those from spiking OPCs produced longer and thicker myelin sheaths around axons.
“We actually developed ‘super cells’ with an even greater capacity to spike than natural cells,” Deng said. “This appears to give them an edge for maturing into oligodendrocytes and producing better myelin.”
It is well known that adult human neural tissue has a poor capacity to regenerate naturally. Although early cells such as OPCs are present, they do not regenerate tissue very effectively when disease or injury strikes.
Deng believes that replacing glial cells with the enhanced spiking OPCs to treat neural injuries and diseases has the potential to be a better strategy than replacing neurons, which tend to be more problematic to work with. Providing the proper structure and environment for neurons to live may be the best approach to regenerate healthy neural tissue. He also notes that many diverse conditions that have not traditionally been considered to be myelin-related diseases – including schizophrenia, epilepsy and amyotrophic lateral sclerosis (ALS) – actually are now recognized to involve defective myelin.