Rules for gene-therapy vectors
Researchers compute, then combine benign viruses to fight disease
Rice University researchers are making strides toward a set of rules to custom-design Lego-like viral capsid proteins for gene therapy.
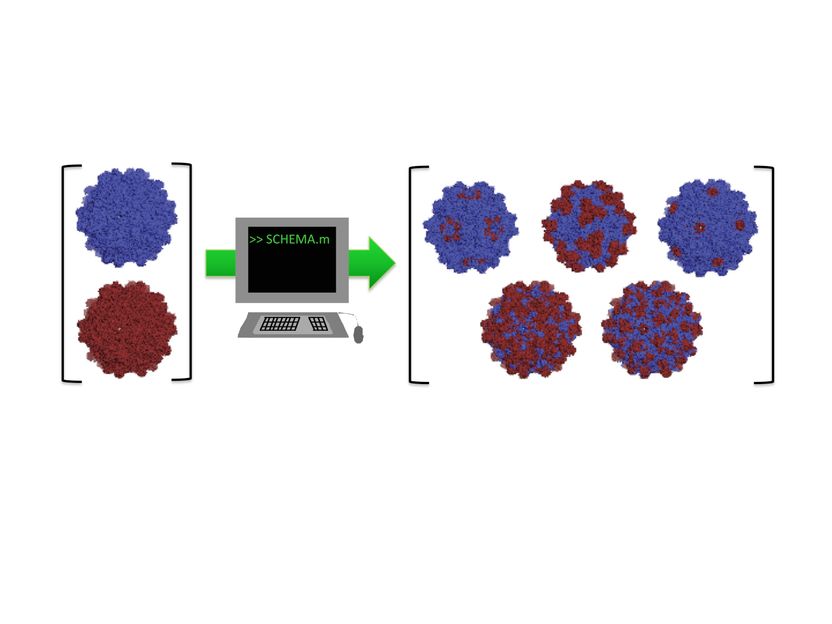
Rice University researchers adapted a computer algorithm to find the parts of two distantly related adeno-associated viruses that could be recombined into new and useful viruses for gene therapy. They intend to determine the rules by which custom viruses can easily be designed for therapies.
Benjamin Adler/Rice
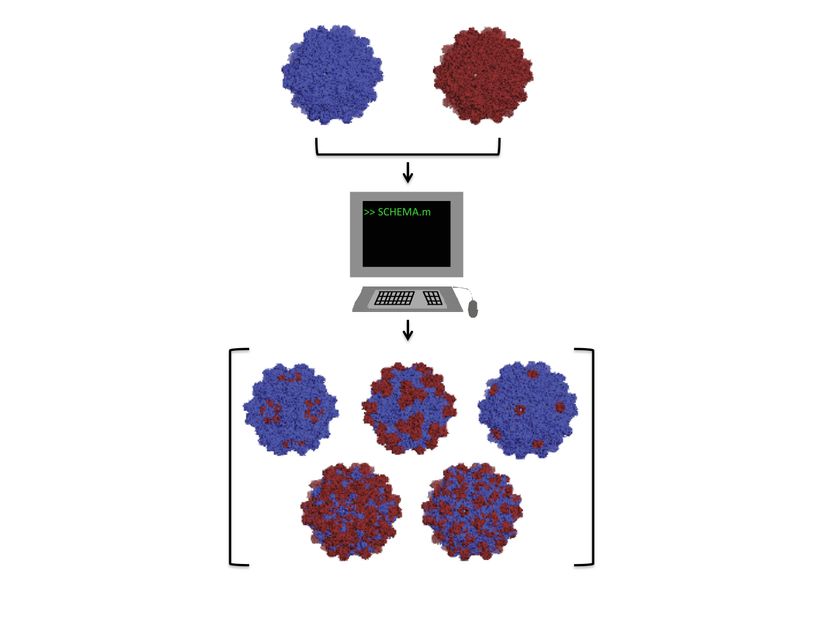
Rice University researchers adapted a computer algorithm to find the parts of two distantly related adeno-associated viruses that could be recombined into new and useful viruses for gene therapy. They intend to determine the rules by which custom viruses can easily be designed for therapies.
Benjamin Adler/Rice
A new paper by Rice scientists Junghae Suh and Jonathan Silberg and their students details their use of computational and bioengineering methods to combine pieces of very different adeno-associated viruses (AAVs) to create new, benign viruses that can deliver DNA payloads to specific cells.
The research appears in the American Chemical Society journal ACS Synthetic Biology.
AAVs are found in nature and commonly infect humans but cause no disease. That makes them good candidates to serve as carriers that target cells and deliver genes to treat diseases.
The team, which included graduate student and lead author Michelle Ho and undergraduates Benjamin Adler and Michael Torre, wants to define rules to design a variety of viruses that deliver therapeutic genes. They used computer models to find likely AAV candidates for recombination and then tested the model predictions by engineering 17 unique virus capsid proteins and evaluating their ability to fold and assemble into capsid-encased viruses.
Gene therapy shows promise in the treatment of not only genetic disorders but also cancer and cardiovascular diseases, said Suh, an assistant professor of bioengineering at Rice’s BioScience Research Collaborative.
“But you need a mechanism to get the correct gene into the human body and to the target cells,” she said. “To do that, people use gene vectors, and viruses encompass the largest category of vectors. They’ve naturally evolved to deliver genes into the body. Our goal is to reprogram them to target specific organs or tissues.
“The big challenge is to go about this in a rational manner,” she said. “People have done a lot of work to solve the structure of viruses. We know what they look like. The question is: How can we use that information to guide the design of our viral vectors?”
The team’s answer starts with the “SCHEMA” algorithm they adapted to predict how parts of very large viruses can recombine by homing in on the viral protein sequences that work well together.
Silberg, an associate professor of biochemistry and cell biology, said approaches to virus design can lean either toward brute force – “Let’s make 1,000 of them and maybe we’ll get lucky” – or purely computational, where a biophysicist will try to predict the role of small changes to the virus capsid.
“We’re working on a hybrid approach,” he said. “Instead of making a random library (of viruses) or computationally designing a single virus, which has a low frequency of working, we’re trying to make smart libraries. We’re learning to adapt computer programs used for small proteins with a few thousand atoms for viruses with more than 100,000 atoms.”
Rather than target mutations in particular viruses, the researchers used the program to compare parts from different but related viruses to see if they would combine together to form new viruses.
“We’re treating them like Legos,” Silberg said. “We’re taking distantly related viruses that nature might not recombine very efficiently and looking for self-contained pieces of these proteins that can be swapped.”
The “parent” viruses were AAV serotype 2, which Suh said is the most commonly studied for gene therapy today, and AAV serotype 4. “They’re part of the same virus family, but genetically, AAV4 is one of the most different from AAV2.”
She said it has been difficult for researchers in the past to rationally make chimeras – one organism that combines parts of two or more genetically distinct elements – from these viruses using traditional techniques.
But Suh’s lab confirmed the chimeric structures predicted by the computer models could be made into real hybrid viruses. Now the challenge is to make a much larger library of chimeric viruses to establish a statistically solid set of guidelines.
“We want to know how to make a more stable virus, or a virus that switches its conformation after it enters a cell,” Silberg said.
“And we want to know how to make one that goes not only just to the brain, but to a specific part of the brain to target a neurodegenerative disease,” Suh added. “The bottom line is that we want these rules.”
Silberg said the researchers had expected to confirm that the SCHEMA algorithm could efficiently predict recombinations that could deliver cargo to cells. “But we also learned something really surprising: that you can beat these viruses up a lot more than you can small proteins, and they still assemble into large virus particles,” he said. “It’s really interesting that viruses fundamentally seem to tolerate the kind of mutation we’re doing.”



































