Atomic insights into plant growth
Researchers from Tübingen resolve how a plant steroid hormone makes plants grow
If one wants to better understand how plants grow, one must analyse the chemistry of life in its molecular detail. Michael Hothorn from the Friedrich-Miescher-Laboratory of the Max Planck Society in Tübingen and his team are doing just that. Their latest work now reveals that a plant membrane receptor requires a helper protein to sense a growth-promoting steroid hormone and to transduce this signal across the cell membrane.
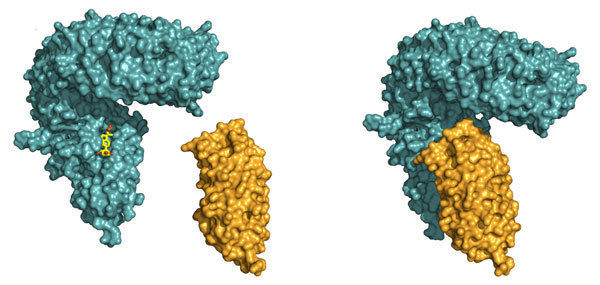
LLR domain of the receptor BRI1 (blue). In a first step, BRI1 binds the steroid hormone (yellow) to a surface pocket. This creates a docking platform for the smaller and shape-complementary LRR-domain of SERK1 (orange), which binds on top, with the steroid acting as a molecular glue.
© J. Santiago
Every cell is surrounded by a greasy cell membrane. Signals from other cells and from the environment must be sensed at the cell surface, transduced across this membrane and translated into a specific response inside the cell. All organisms have evolved membrane receptor proteins to get these complex tasks done, but plant membrane receptors look drastically different from the well-studied players in animals and bacteria. The plant steroid receptor BRI1, which can sense a small steroid hormone promoting plant growth, belongs to the family of leucine-rich repeat (LRR) receptor kinases, which are responsible for most membrane signalling events in plants. It was previously shown that BRI1 directly binds the small steroid hormone with its LRR-domain at the cell surface.
Julia Santiago, a postdoctoral fellow in the Hothorn lab, could now demonstrate that BRI1 requires a helper protein to correctly sense the hormone and transduce the signal across the membrane. The helper SERK1 is a known player in the brassinosteroid signalling pathway, but it came as a surprise to see how early on it is required. By hitting protein crystals containing the ternary BRI1 – steroid hormone – SERK1 complex with intense X-rays, Santiago could see that SERK1 contributes directly to the formation of the hormone binding pocket, with both proteins interacting with the hormone. The steroid thus acts as a molecular glue which promotes association of the BRI1 and SERK1 LRR domains at the cell surface. This then causes interaction of the cytoplasmic kinases domains in the cell interior, which in turn activates a well characterized signaling pathway triggering the growth response.
The interesting feature of SERK1 is that it can help activate several seemingly unrelated plant receptor kinases, which bind vastly different ligands and trigger different responses. The new structures provide a first glimpse on how SERK1 might be able to do that. Instead of shaking hands with BRI1, it only uses a few 'finger tips' to contact the receptor. Other, strictly conserved surface patches remain available for the interaction with other plant receptor kinases and, potentially their ligands. “There must be some advantage to having all this different functions combined into a single helper protein”, Hothorn speculates. Notably, the use of a shared helper protein could allow different signaling pathway to communicate with each other.
The atomic models offer other novel insights too: “Looking at our models, we can now predict pretty well, which mutation in the receptor or helper protein should have an effect on the down-stream signaling pathway. We also know what parts of the hormone are really important to make it bind to the receptor or to the helper protein”. Such detailed insights may promote the rational design of synthetic plant steroid hormones and receptor antagonist with applications in basic research, and perhaps someday in the field.
Original publication
Julia Santiago, Christine Henzler, Michael Hothorn Molecular mechanism for plant steroid receptor activation by somatic embryogenesis co-receptor kinases Science Express; 9 August, 2013
Original publication
Julia Santiago, Christine Henzler, Michael Hothorn Molecular mechanism for plant steroid receptor activation by somatic embryogenesis co-receptor kinases Science Express; 9 August, 2013
Topics
Organizations
Other news from the department science

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Harold_Ridley_(ophthalmologist)
Evergreens and hot topics: ACHEMA preparations shift into high gear

ST Instruments B.V. - Shedrecht, Netherlands
-phob-
Innate immunity - When DNA is out of place
Protein that delays cell division in bacteria may lead to the identification of new antibiotics
Brain Insulin Sensitivity Determines Body Weight and Fat Distribution - High insulin sensitivity associated with reduction in visceral fat and weight
Australian_Doctors_for_Africa
Toxic_oil_syndrome
Stanford-spawned nanoparticles home in on brain tumors, boost accuracy of surgical removal
Neuroscientists posit that brain region is a key locus of learning