Protein team produces molecular barrels
Researchers show that two protein machineries collaborate on the development of barrel structures in the mitochondria
Advertisement
Research groups headed by Prof. Dr. Nikolaus Pfanner, Dr. Nils Wiedemann, and Dr. Thomas Becker from the University of Freiburg and their colleagues have demonstrated how molecular protein barrels form in the outer membrane of the mitochondria, the powerhouses of the cell. Their studies revealed that two protein machineries cooperate in an unexpected way.
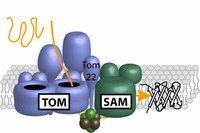
The protein machineries TOM und SAM are linked via Tom22 and work together in the maturation process of beta-barrel structures of proteins. Modified from Becker et al., 2008; Biochim. Biophys. Acta 1777, 447-563
© Thomas Becker (BBA-2008)
Mitochondria are essential for the survival of the cell, rendering such vital services as providing the energy for cell metabolism. Mitochondria are surrounded by two membranes. The outer membrane contains characteristic proteins with a barrel-like structure, the beta-barrel structure. These proteins extend across the membrane and are crucial for the transport of proteins and metabolic products into the mitochondria. The proteins are produced as precursors in the cytosol, only forming their mature barrel structure upon entering the mitochondrion. They are imported through the pores of the protein complex TOM, the translocase of the outer mitochondrial membrane, and then transported to a second protein machinery in the outer membrane, the sorting and assembly machinery SAM. Finally, the SAM complex integrates the proteins into the membrane. The individual steps leading to the formation of the beta-barrel structure and the transfer of the precursor protein from TOM to SAM were not previously understood.
The researchers studied the formation of the beta-barrel structure within the context of a partnership between the Collaborative Research Center 746 “Functional Specificity by Coupling and Modification of Proteins,” the Cluster of Excellence BIOSS Centre for Biological Signalling Studies, and the Spemann Graduate School of Biology and Medicine.
The team headed by Nils Wiedemann demonstrated that the beta-barrel structure forms at the SAM complex. The PhD student Jian Qiu discovered that the receptor protein Tom22 plays a key role in this process. This comes as a surprise, because it was previously thought that TOM and SAM were independent protein machineries. However, findings from Thomas Becker’s research group showed that the two complexes directly interact with each other. They are linked by Tom22, as the PhD student Lena-Sophie Wenz discovered. If Tom22 is not present, the molecular bridge between TOM and SAM is lost – severely hampering the formation of beta-barrel structures. The findings of this study demonstrate that the direct transfer of the imported protein from the TOM complex to the SAM complex enables an efficient formation of mitochondrial beta-barrel structures.