Biobatteries catch breath
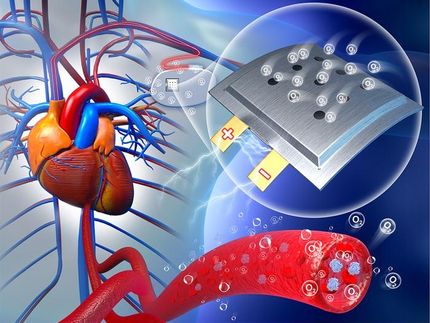
An air-breathing bio-battery has been constructed by researchers from the Institute of Physical Chemistry of the Polish Academy of Sciences in Warsaw. The core element providing the new power source with relatively high voltage and long lifetime is a carefully designed cathode taking up oxygen from air and composed of an enzyme, carbon nanotubes and silicate.
People are increasingly taking advantage of devices supporting various functions of our bodies. Today they include cardiac pacemakers or hearing aids; tomorrow it will be contact lenses with automatically changing focal length or computer-controlled displays generating images directly in the eye. None of these devices will work if not coupled to an efficient and long-lasting power supply source. The best solution seems to be miniaturised biofuel cells consuming substances naturally occurring in human body or in its direct surrounding.
Researchers from the Institute of Physical Chemistry of the Polish Academy of Sciences (IPC PAS) in Warsaw developed an efficient electrode for the use in construction of biofuel cells or zinc-oxygen biobatteries. After installation in a cell, the new biocathode generates a voltage, during many hours, that is higher than that obtained in existing power sources of similar design. The most interesting is that the device is air-breathing: it works at full efficiency when it can take oxygen directly from the air.
Common batteries and rechargeable batteries are unsuitable to power implants inside the human body as they use strong bases or acids. These agents can on no account get into the body. The battery housing must be therefore absolutely tight. But in line with reducing the battery size, it must be better isolated. In extreme cases, the weight of the housing of a common, miniaturised battery would be even a few dozen times greater than the weight of the battery's active components that generate electricity. And here biofuel cells offer an essential advantage: they do not require housing. To get electricity, it is enough to insert the electrodes into the body.
"One of the most popular experiments in electrochemistry is to make a battery by sticking appropriately selected electrodes into a potato. We are doing something similar, the difference is that we are focusing on biofuel cells and the improvement of the cathode. And, of course, to have the whole project working, we'd rather replace the potato with... a human being", says Dr Martin Jönsson-Niedziółka (IPC PAS).
In the experiments, Dr Jönsson-Niedziółka's group uses zinc-oxygen batteries. The principle of their operation is not new. The batteries constructed in this way had been popular before the time of alkaline power sources came. "At present, many laboratories work on glucose-oxygen biofuel cells. In the best case they generate a voltage of 0.6-0.7 V. A zinc-oxygen biobattery with our cathode is able to generate 1.75 V for many hours.", says Adrianna Złoczewska, a PhD student at the IPC PAS, whose research has been supported under the International PhD Projects Programme of the Foundation for Polish Science.
The main component of the biocathode developed at the IPC PAS is an enzyme surrounded by carbon nanotubes and encapsulated in a porous structure – a silicate matrix deposited on an oxygen permeable membrane. "Our group had been working for many years on techniques that were necessary to construct the cathode using enzymes, carbon nanotubes and silicate matrices", stresses Prof. Marcin Opałło (IPC PAS).
An electrode so constructed is installed in a wall of a small container. To have the biofuel cell working, it is enough to pour an electrolyte (here: a solution containing hydrogen ions) and insert the zinc electrode in the electrolyte. The pores in the silicate matrix enable oxygen supply from the air and H+ ions from the solution to active centres of the enzyme, where oxygen reduction takes place. Carbon nanotubes facilitate transport of electrons from the surface of the semipermeable membrane.
A cell with the new biocathode is able to supply power with a voltage of 1.6 V, for a minimum one and a half of a week. The cell efficiency decreases with time, likely because of gradual deactivation of the enzyme on the biocathode. "Here not everything is dependent on us, but on the progress in biotechnology. The lifetime of a biofuel cells with our biocathode could be significantly prolonged, if the enzyme regeneration processes are successfully developed", says Dr Jönsson-Niedziółka.
In the experiments carried out so far, a stack of four batteries connected in series successfully powered a lamp composed of two LEDs. Before, however, the biofuel cells based on the design developed at the IPC PAS get popularised, the researchers must solve the problem of relatively low electric power that is common to all types of biofuel cells.
The research presented here is important not only in view of the miniaturisation of power supply sources for medical implants, biosensors or light-emitting tattoos. The processes responsible for electricity generation in biofuel cells are potentially suitable for use in electric power generation in a larger scale. The limiting factors here are the properties of the enzymes, so that further advancement in this area is essentially dependent on the development of the biotechnology.