European Medicines Agency update on Diane 35 and generics used in the treatment of acne
The French medicines agency announced its plan to suspend the marketing authorisation for Diane 35 and its generics for acne treatment in France.
These medicines are widely used across Europe. They have been authorised at the level of individual Member States for many years. In France, they are only authorised for the treatment of acne, but in a number of other Member States they are also authorised for the treatment of acne in women who wish to receive oral contraception, as well as for the treatment of other skin conditions.
The announcement in France follows a review by the French medicines agency (ANSM) of known data. ANSM considered that Diane 35 and its generics carry a risk of thromboembolism which has been well known for many years, while their effectiveness in treating acne was only moderate and alternative treatments for acne are available. In addition, it noted that they are widely used off-label as a contraceptive.
Although Member States can take unilateral action to suspend the marketing authorisation of a medicine, European legislation requires that there is a coordinated European approach in these instances. France has already indicated that it will ask the European Medicines Agency to carry out a European-wide review of Diane 35 and its generics. Once the notification has been received, the Agency’s Pharmacovigilance Risk Assessment Committee (PRAC) will evaluate all evidence on the benefits and risks of these medicines and give a recommendation on whether their marketing authorisations should be varied, suspended or revoked, in the interest of all patients in the EU.
Pending the outcome of the PRAC review, women who are currently taking Diane 35 or one of its generics are advised not to stop the medicine. If a woman has concerns, she can discuss this with her doctor.
Other news from the department manufacturing

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
YM Biosciences Reports Additional Results From Nimotuzumab Phase III Study in Children With Glioma
In the bacterial world of your mouth, nurture wins out over nature
Gerhard Schratt, PhD and Professor Albert Sickmann receive the Analytica Research Prize
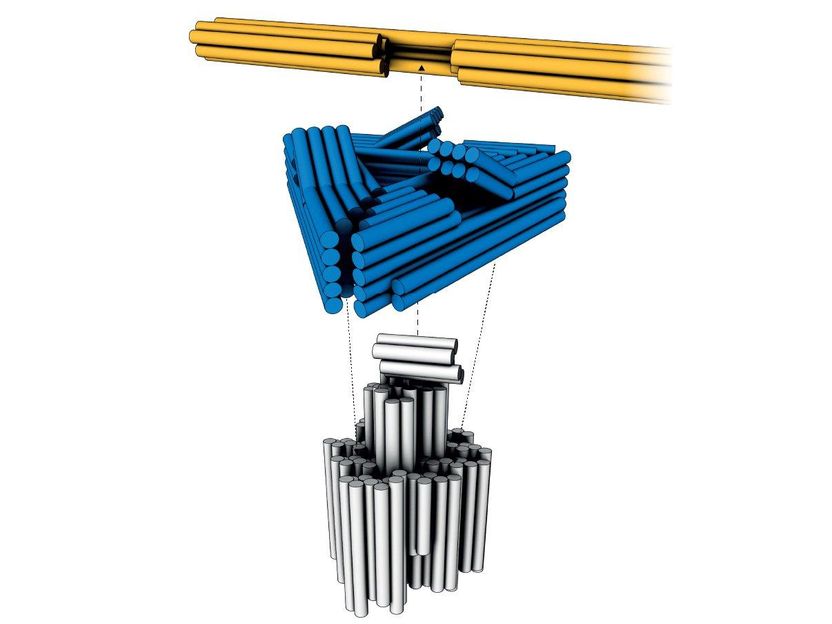
First electric nanomotor made from DNA material - Synthetic rotary motors at the nanoscale perform mechanical work
List_of_drugs:_Meu-Mi
TGen and Affymetrix Partner to Use GeneChip(R) DNA Analysis Products in Major Collaborative Programs - Genotyping Technology that Leverages an Innovative Whole-Genome SNP Assay Is Available to Commercial and Academic Research Partners and Future Collaborators
Stem Cell Innovations AND TWente University to evaluate bone forming capacity of Pluricells
Pfizer will invest €90.5 million in Valneva - Valneva and Pfizer Enter into an Equity Subscription Agreement and Update Terms of Collaboration Agreement for Lyme Disease Vaccine Candidate VLA15

Demonstration of large-scale technique to produce quantum dots
American Peptide Company expands its manufacturing and service portfolio
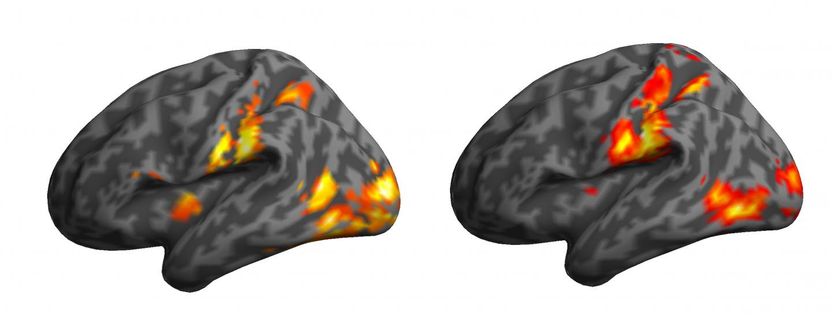
Sometimes just watching hurts - and the signs of pain are seen in the brain




















































