Ready. Get set. Repress!
Stowers scientists manipulate the Set2 pathway to show how genes are faithfully copied
The first step in gene expression is the exact copying of a segment of DNA by the enzyme known as RNA polymerase II, or pol II, into a mirror image RNA. Scientists recognize that pol II does not transcribe RNA via a smooth glide down the DNA highway but instead encounters an obstacle course of DNA tightly wound around barrier proteins called histones. Those proteins must be shoved aside for pol II to trundle through.
Histone reshuffling plays an important role in regulating gene expression.
Courtesy of Dr. Swami Venkatesh, Stowers Institute for Medical Research
Previous work from the lab of Jerry Workman, Ph.D., an investigator at the Stowers Institute for Medical Research, showed how a protein associated with pol II called Set2 biochemically restored an inhibitory histone landscape once a round of transcription was completed in order to prevent expression of potentially harmful RNA snippets. Now in a study published in the August 22, 2012, advance online issue of Nature, his group uses yeast mutants in Set2 to reveal a surprising mechanism cells use to keep gene expression going—both in appropriate circumstances and inappropriate ones, as in cancer cells.
"The goal of my lab is to understand is how transcription works," says Workman. "We knew that synthesis of an RNA strand must start at the beginning of a gene for it to ultimately code for a full-length protein and that it was essential to prevent RNA synthesis beginning at cryptic sites within a gene. In this study we addressed how that could happen on the molecular level."
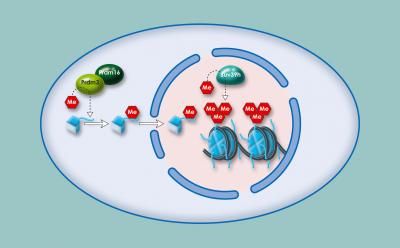
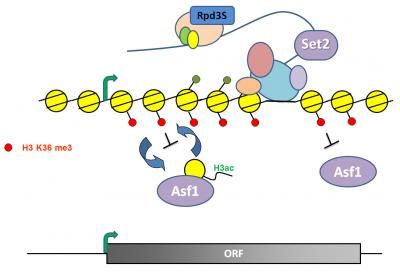
Two classes of chromatin remodeling factors vie to activate or repress gene expression. The "activators" pry histones from DNA to allow passage of pol II by decorating histones with acetyl groups, which loosens their grip on DNA. The opposing team, which includes Set2, then terminates gene expression by planting their own biochemical flag, this one a methyl group, which then attracts an enzyme to snip off the acetyl groups and restore chromatin to an inaccessible state. This repression assures that erroneous initiation of transcription does not occur at a so-called "cryptic" site within a gene.
Previously, many assumed that histones remain in contact with a DNA strand while they were decorated or stripped of acetyl groups by chromatin remodelers. "That was one possible scenario but we simply did not know what happened to histones on chromatin as the RNA strand elongated," says Swami Venkatesh, Ph.D., a postdoctoral researcher in the Workman lab and the study's first author. "We thought that some kind of exchange of histones took place during transcription but weren't sure whether it happens over an entire gene."
To investigate potential histone reshuffling, the group assessed chemical modification of histones in a form of the brewer's yeast Saccharomyces cerevisiae engineered to track whether labeled histones moved in and out of chromatin. Using this system they compared global acetylation and methylation of histones in all 6000 genes in both Set2 mutants and normal yeast.
They first observed that in normal cells the inhibitory methylation flag planted by Set2 spanned the trajectory of genes being expressed and that mark was, as anticipated, missing in Set2 mutants. In those mutants a large portion of genes showed a reciprocal increase in the acetylation mark, which could be explained by the loss of Set2's recruitment of the acetyl snipper.
Further analysis revealed many of those acetylated histones had not in fact remained embedded in chromatin but had instead been "imported" from outside stores or as Venkatesh says, "pre-acetylated." "This is important because it means that you don't need to bring in a remodeling enzyme to re-acetylate histones," he says.
These studies also reveal that Set2 plays a more complex role in controlling gene expression than anticipated. "The methyl mark placed on the histones by Set2 serves two functions," explains Workman. "It not only recruits the de-acetylating enzyme that removes the acetyl marks from histones, but these new findings suggest it also prevents incorporation of pre-acetylated histones on the gene."
The group also correlated these chemical modifications to aberrant gene expression using microarray analysis, which showed that unlike normal yeast, Set2 mutants produce variously truncated "cryptic" RNA transcripts.
"As genes are expressed, you do not want to shunt the system to produce RNAs that cannot be used," says Venkatesh. "When cells undergo heat or environmental stress, they respond by making short RNAs encoding proteins that send a stress signal. Cells need Set2 under normal situations to keep these cryptic transcription start sites quiet."
Workman agrees, noting that truncated strands of RNA could possibly interfere with translation of normal RNAs into protein. "The fact that they are generated indicates chromosomal instability in the area encompassing these genes, a situation which can lead to cancer in humans," he says. "The human counterpart of the Set2 protein, SETD2, reportedly functions as a tumor suppressor and its activity is lost in several types of cancers, including breast and renal carcinoma."
Recognizing that histone exchange actually occurs over a large proportion is also relevant to cancer therapies in the pipeline. "Proteins that both acetylate and deacetylate histones are under investigation as targets of anti-cancer drugs," says Venkatesh. "So it is very important to know how acetylated histones are brought to a gene. Mechanistic studies like ours provide this kind of insight."