MagForce to radically enforce market set up strategy with new management team
Core functions will be centralized in Munich
MagForce AG announced additions to the management board and further measures to enforce commercialization of NanoTherm® therapy. Thus, focussing on establishing the NanoTherm® therapy in the oncology market as well as on its commercialization for near-term value generation.
In the short to mid term, MagForce will dedicate its financial resources to the post marketing trial in glioblastoma, which is expected to start in early 2013 and is supposed to make a significant contribution to its strategic set up. Simultaneously, the Company will also focus on the commercialization of its NanoTherm® therapy with its distribution partners including DELRUS and TekGrup, development partners such as the Mayo Clinic for gastro-intestinal cancer and the Department of Urology at Duesseldorf University for prostate cancer, as well as the production of nanoparticles.
In line with this strategy, the Company's core functions, including clinical and business development, medical affairs plus legal & IP will be concentrated at the MagForce site in Munich where these teams are already located. Production of nanoparticles, NanoActivators(TM), software engineering as well as the finance department will remain at the Company's registered office in Berlin. All early research activities will be reduced to a minimum and will be outsourced as deemed neccessary.
In this respect experienced senior executives are added to the management board. Prof Dr Hoda Tawfik, currently VP Clinical Development and Medical Affairs, will be appointed COO. Christian von Volkmann, currently acting CFO, will assume the position of CFO, both effective immediately. They will jointly lead the Company as co-CEOs going forward. The founder and long-term Board member Dr Andreas Jordan will step down from the management board of MagForce to pursue new projects, but will continue to serve the Company as an advisor.
Along with these measures the headcount of MagForce will be reduced from 27 to 12. These measures are meant to annually save the Company a seven digit Euro amount.
Other news from the department business & finance
These products might interest you

Eclipse by Wyatt Technology
FFF-MALS system for separation and characterization of macromolecules and nanoparticles
The latest and most innovative FFF system designed for highest usability, robustness and data quality

DynaPro Plate Reader III by Wyatt Technology
Screening of biopharmaceuticals and proteins with high-throughput dynamic light scattering (DLS)
Efficiently characterize your sample quality and stability from lead discovery to quality control

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Category:Polysaccharides
Louis_Harold_Gray
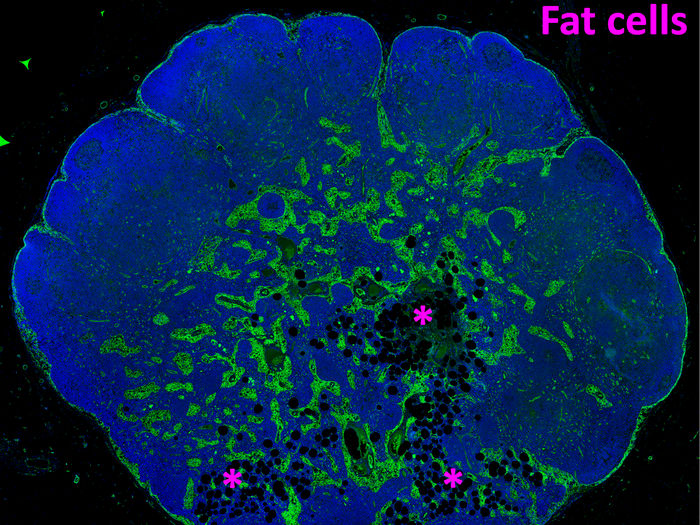
How fat takes over the lymph nodes as we age
Cellulose_sulfate
Category:Polysaccharide_encapsulated_bacteria
Syntaxin enters manufacturing agreement with SynCo Bio Partners B.V - Progresses Acromegaly programme towards clinic
ClinPhone Invests in IT Infrastructure to Make Customer Clinical Trial Data Ultra Secure
Cystic_medial_necrosis
SymbioTec signs representation contract with Tytonis
Philip_Showalter_Hench