Finished heart switches stem cells off
Transcription factor Ajuba regulates stem cell activity in the heart during embryonic development
It is not unusual for babies to be born with congenital heart defects. This is because the development of the heart in the embryo is a process which is not only extremely complex, but also error-prone. Scientists from the Max Planck Institute for Heart and Lung Research in Bad Nauheim have now identified a key molecule that plays a central role in regulating the function of stem cells in the heart. As a result, not only could congenital heart defects be avoided in future, but new ways of stimulating the regeneration of damaged hearts in adults may be opened up.
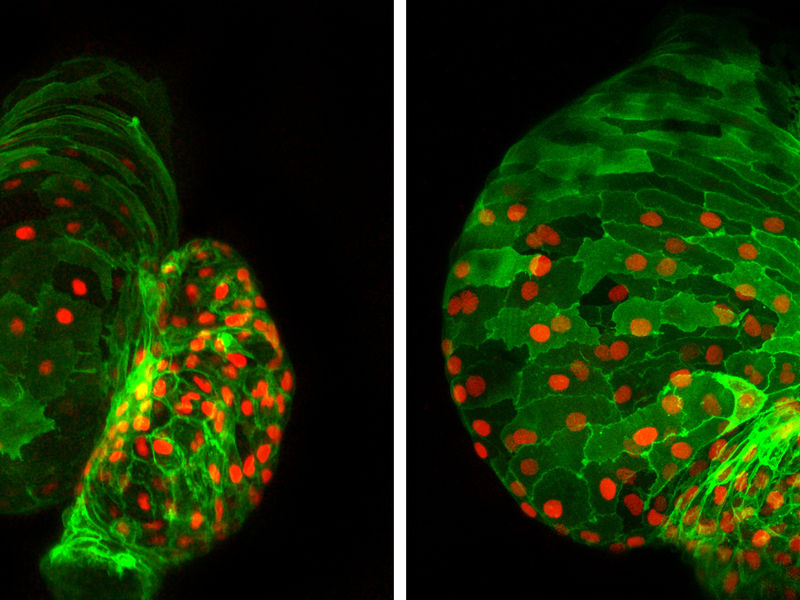
Cardiac development out of control: Absence of the transcription factor Ajuba during cardiac development, as is the case in the right-hand photo due to genetic intervention, disrupts development of the heart in the fish embryo. In addition to an increased number of cardiac muscle cells (green with red-stained nuclei), the heart is additionally deformed during development.
© Max Planck Institute for Heart and Lung Research
It's a long road from a cluster of cells to a finished heart. Cell division transforms what starts out as a collection of only a few cardiac stem cells into an ever-larger structure from which the various parts of the heart, such as ventricles, atria, valves and coronary vessels, develop. This involves the stem and precursor cells undergoing a complex process which, in addition to tightly regulated cell division, also includes cell migration, differentiation and specialisation. Once the heart is complete, the stem cells are finally switched off.
Scientists from the Max Planck Institute for Heart and Lung Research in Bad Nauheim have now discovered how major parts of this development process are regulated. Their search initially focused on finding binding partners for transcription factor Isl1. Isl1 is characteristic of a specific group of cardiac stem cells which are consequently also known as Isl1+ cells. During their search, the researchers came across Ajuba, a transcription factor from the group of LIM proteins. "We then took a closer a look at the interaction between these two molecules and came to the conclusion that Ajuba must be an important switch", says Gergana Dobreva, head of the "Origin of Cardiac Cell Lineages" Research Group at the Bad Nauheim-based Max Planck Institute.
Using an animal model, the scientists then investigated the effects of a defective switch on cardiac development. Embryonic development can be investigated particularly effectively in the zebrafish. The Bad Nauheim-based researchers therefore produced a genetically modified fish that lacked a functioning Ajuba protein. Cardiac development in these fishes was in fact severely disrupted. In addition to deformation of the heart, caused by twisting of the cardiac axis, what particularly struck the researchers was a difference in size in comparison with control animals. "In almost all the investigated fish we observed a dramatic enlargement of the heart. If Ajuba is absent, there is clearly no other switch that finally silences the Isl1-controlled part of cardiac development", says Dobreva.
Further investigations revealed that the enlargement of the heart is in fact attributable to a greatly increased number of cardiac muscle cells. The reason for this was in turn that the number of Isl1+ cells, i.e. the cardiac muscle precursor cells, was distinctly raised right from an early phase of development. Ajuba is a decisive factor in controlling stem cell activity: it binds to Isl1 molecules, thus blocking their stimulant effect.
The results from the study could have potential future applications. "Once we understand how cardiac development is regulated, we will also be more familiar with the causes of congenital heart defects and will consequently be able to consider therapeutic approaches", comments Dobreva. Damaged adult hearts can also be repaired in this way: "One possibility would be to optimise the production of replacement cells from embryonic or artificially produced stem cells in the laboratory. Silencing Ajuba in these cells might enhance their development into functional cardiac muscle cells. Sufficient replacement cells for treating patients could be cultured in this way." Another possibility is to stimulate stem cell activity by silencing Ajuba in the damaged heart and so cause the heart to regenerate itself. Further studies are now set to investigate how feasible this might be.