Biotest starts combination therapy study with BT-062 in multiple myeloma
Biotest AG is pursuing an innovative therapeutic strategy to treat multiple myeloma using the antibody-drug conjugate (ADC) BT-062. BT-062 is currently in clinical development for treating patients with relapsed or relapsed/refractory multiple myeloma. Two clinical trials investigating different dose schedules of BT-062 as monotherapy have shown good tolerability and have provided evidence for anti-tumor activity.
Whereas treatment of 32 patients within the first monotherapy study 969 is finished, Biotest continues to investigate BT-062 as monotherapy in study 975. In study 975 about 50 patients with relapsed or relapsed/refractory multiple will be treated at a more frequent dose schedule, receiving intravenous administration of BT-062 on days 1, 8, and 15 every 4 weeks. The patient recruitment of the first seven dose levels has been completed. So far BT-062 continued to be well tolerated. In addition, initial evidence of efficacy was confirmed.
Combination therapies are widely used to treat multiple myeloma and other cancers to improve overall response rates. Preclinical studies using in vitro and in vivo animal models show a strong increase of efficacy when BT-062 is combined with widely used multiple myeloma drugs such as lenalidomide, suggesting an additive or even synergistic anti-tumor effect of such combinations in patients.
Currently, Biotest expands the clinical development of BT-062 into combination therapy. The phase I/IIa study (study no. 983) investigates safety and efficacy of BT-062 when administered on days 1, 8, and 15 every 4 weeks in combination with lenalidomide and dexamethasone in patients with relapsed or relapsed/refractory multiple myeloma.A few days ago the first patient in study 983 started treatment.
Additionally, Biotest will receive funding to evaluate BT-062 in preclinical solid tumor models by the center of excellence cluster CI3 Rhein-Main 'Individualized ImmuneIntervention'. CI3 is a cluster initiative of more than 100 partners of universities, biotechnology start-ups and pharmaceutical industry, which is supported by the German Federal Ministry of Education and Research.
Biotest will continue to focus its resources on the development of BT-062 in the lead indication multiple myeloma. For further clinical development in solid tumor indications Biotest intends to collaborate with a strategic partner.
Most read news
Other news from the department research and development

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Arrhythmogenic_right_ventricular_dysplasia
Amplification_(psychology)
ChemMaps lets researchers navigate the chemical universe
American_Synesthesia_Association
Mary_Ainsworth
Category:Tetracyclic_antidepressants
-phob-
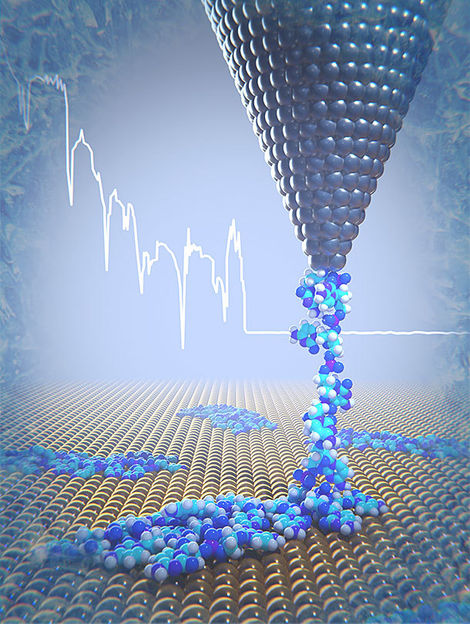
Cryo-force spectroscopy reveals the mechanical properties of DNA components
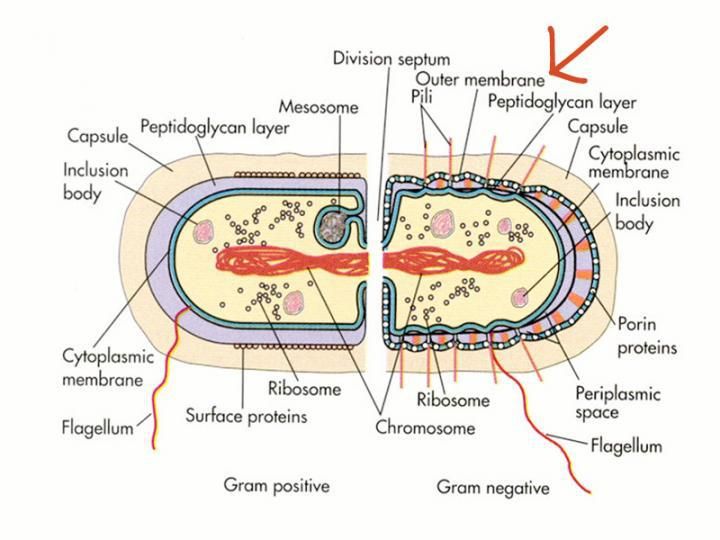
Know your enemy - An arsenal to fight antibiotic-resistant bacteria
Peptides vs. superbugs