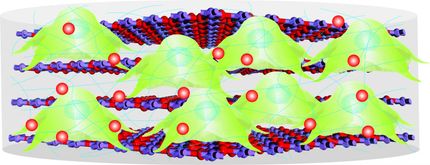
Stem-cell-growing surface enables bone repair
University of Michigan researchers have proven that a special surface, free of biological contaminants, allows adult-derived stem cells to thrive and transform into multiple cell types. Their success brings stem cell therapies another step closer.
To prove the cells' regenerative powers, bone cells grown on this surface were then transplanted into holes in the skulls of mice, producing four times as much new bone growth as in the mice without the extra bone cells.
An embryo's cells really can be anything they want to be when they grow up: organs, nerves, skin, bone, any type of human cell. Adult-derived "induced" stem cells can do this and better. Because the source cells can come from the patient, they are perfectly compatible for medical treatments.
In order to make them, Paul Krebsbach, professor of biological and materials sciences at the U-M School of Dentistry, said, "We turn back the clock, in a way. We're taking a specialized adult cell and genetically reprogramming it, so it behaves like a more primitive cell."
Specifically, they turn human skin cells into stem cells. Less than five years after the discovery of this method, researchers still don't know precisely how it works, but the process involves adding proteins that can turn genes on and off to the adult cells.
Before stem cells can be used to make repairs in the body, they must be grown and directed into becoming the desired cell type. Researchers typically use surfaces of animal cells and proteins for stem cell habitats, but these gels are expensive to make, and batches vary depending on the individual animal.
"You don't really know what's in there," said Joerg Lahann associate professor of chemical engineering and biomedical engineering.
For example, he said that human cells are often grown over mouse cells, but they can go a little native, beginning to produce some mouse proteins that may invite an attack by a patient's immune system.
The polymer gel created by Lahann and his colleagues in 2010 avoids these problems because researchers are able to control all of the gel's ingredients and how they combine.
"It's basically the ease of a plastic dish," said Lahann. "There is no biological contamination that could potentially influence your human stem cells."
Lahann and colleagues had shown that these surfaces could grow embryonic stem cells. Now, Lahann has teamed up with Krebsbach's team to show that the polymer surface can also support the growth of the more medically promising induced stem cells, keeping them in their high-potential state. To prove that the cells could transform into different types, the team turned them into fat, cartilage and bone cells.
They then tested whether these cells could help the body to make repairs. Specifically, they attempted to repair five-millimeter holes in the skulls of mice. The weak immune systems of the mice didn't attack the human bone cells, allowing the cells to help fill in the hole.
After eight weeks, the mice that had received the bone cells had 4.2 times as much new bone, as well as the beginnings of marrow cavities. The team could prove that the extra bone growth came from the added cells because it was human bone.
"The concept is not specific to bone," said Krebsbach. "If we truly develop ways to grow these cells without mouse or animal products, eventually other scientists around the world could generate their tissue of interest."
In the future, Lahann's team wants to explore using their gel to grow stem cells and specialized cells in different physical shapes, such as a bone-like structure or a nerve-like microfiber.
Most read news
Organizations
Other news from the department science

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
CHARGE_syndrome
Stretched, ordered DNA molecules could bring insights into disease
Study uncovers new hurdle for developing immunotherapies
How a molecular Superman protects the genome from damage - Scientists find a new role for RNAi protein Dicer in preventing collisions during DNA replication
Networks of Gene Activity Control Organ Development
AIDS_advocacy
Evotec completes acquisition of Rigenerand