Medicyte Awarded ISO 9001 Certification
Offering a new and unique technology to propagate primary human cells to virtually unlimited numbers, Medicyte has now gone a step further in its high quality policy.
As announced, its quality management system was certified in December 2011 to meet the criteria of the globally recognized standard DIN ISO 9001:2008, supervised by the TÜV. The certification was performed in order to meet the strict quality requirements of customers in the pharmaceutical, chemical and cosmetic industries. It underlines Medicyte’s consistency and continuous endeavours to improve the quality of products and customer service. The certificate covers both the controlled and scalable production and sales process of human quasi-primary cell products. This is especially important for the supply of human cells, such as hepatocytes and keratinocytes, for ADME/Tox testing, which should be consistently of high quality.
"We have always had a strict total quality system, but the additional structure of the DIN ISO 9001 registration has helped us to improve and standardize our internal daily tasks and thus our customers’ satisfaction with our products and services. Becoming an ISO-certified company is a natural extension of our quality commitment. Customers can feel confident relying on our compliance for quality standards of our products and services because they are aware that we follow internationally accepted standards,” said Nadja Hartmann, Medicyte’s Total Quality Manager.
Most read news
Other news from the department politics & laws

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
More news from our other portals
Last viewed contents

Trimaco Systeme GmbH - Zürich, Switzerland

Lumiphore, Inc. - Richmond, USA
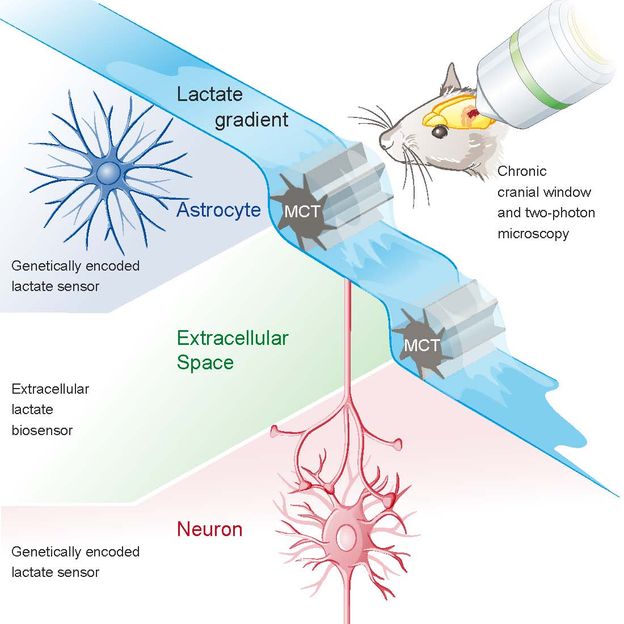
Lactate for Brain Energy
List_of_bolete_species
CureVac Receives U.S. Patent Covering Entire Process for Pharmaceutical Manufacturing of RNA
Casting a wide net to fight coronaviruses
Sexual transmission of Ebola likely to impact course of outbreaks
How a protein in your brain could protect against Alzheimer's disease - New research sets the stage for new therapeutic strategies for Alzheimer's disease
New model finds HIV acute phase infectivity may be lower than previously estimated

KNAUER is expanding its business activities into the field of lipid nanoparticle production equipment - In connection with the development of mRNA-based vaccines against Corona, LNPs have shown to be a suitable delivery form




















































