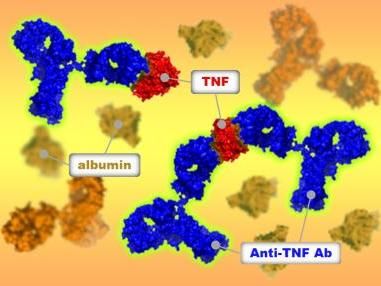
Cimzia shows positive top-line phase 3 results in axial spondyloarthritis and ankylosing spondylitis
UCB announced positive top-line results from the phase 3 study designed to evaluate the efficacy and safety of certolizumab pegol in patients with adult-onset active axial spondyloarthritis (AxSpA), a family of inflammatory rheumatic diseases which includes ankylosing spondylitis (AS).
"The population in this study included both patients with AS and with an early stage of the disease, called non-radiographic axial spondyloarthritis. Both populations are part of a recently defined group of rheumatic diseases, axial spondyloarthritis. The positive top-line results are very encouraging since there is a need for treatments for patients with non-radiographic AxSpA and for additional effective anti-TNF treatments for AS," said Professor Dr Iris Loew-Friedrich, Chief Medical Officer and Executive Vice President UCB. "Full analysis of the efficacy and safety data of this study is on-going and we look forward to discussing the results with the regulatory authorities.”
In this 24-week, multicenter, double-blind, placebo-controlled phase 3 study, 325 patients with AxSpA were randomized to receive certolizumab pegol, 200 mg every two weeks, or 400 mg every four weeks, or placebo. This dosing schedule followed a loading dose of certolizumab pegol (400 mg) at weeks 0, 2 and 4. At week 12, a statistically significant higher proportion of patients receiving certolizumab pegol compared to those receiving placebo achieved the primary endpoint of at least 20 per cent change in the Assessment of SpondyloArthritis international Society improvement criteria (ASAS20). Sub-population analyses indicated that certolizumab pegol also improved the signs and symptoms of AS and non-radiographic AxSpA, pre-specified sub-groups of the overall population.
The most common treatment emergent adverse events that occurred in = 5% of patients taking certolizumab pegol (all doses) and more frequently than placebo were upper respiratory tract infection, elevated liver function tests and abnormal CPK levels. While the incidence of serious treatment emergent adverse events reported in the certolizumab group was comparable to the placebo group, the incidence of serious infections/infestations was higher in the certolizumab pegol group.
The study’s primary endpoint was the ASAS20 improvement criteria and is defined as an improvement of at least 20% and absolute improvement of at least 1 unit on a 0-10 scale in at least three of the four following domains: patient global assessment, pain assessment, patient function, and inflammation and the absence of deterioration in the remaining domain.
Most read news
Topics
Organizations
Other news from the department research and development

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Urinary tract infection: How bacteria nestle in
Researchers computationally find the needle in a haystack to treat rare diseases
DNA guardians out of control - Our own immune system can become the enemy when mechanisms that are actually protective get out of control
Clinical trial uses cold virus to attack bladder cancer
Researchers engineer hardier microbes to improve bioproduction of fuels, chemicals