Convergence Pharmaceuticals Announces Start of Phase II Study for CNV1014802 for Trigeminal Neuralgia
Convergence Pharmaceuticals Limited announced that the Phase II proof of concept study for CNV1014802 for the treatment of pain associated with trigeminal neuralgia (TN) has started. CNV1014802 is novel small molecule, state-dependent sodium channel blocker that exhibits potency and selectivity against the Nav1.7 sodium channel.
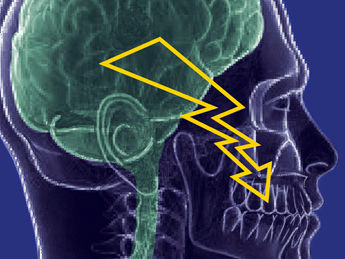
TN is a very severe form of facial pain that is experienced in short bursts or attacks. The International Association for the Study of Pain (IASP) defines TN as sudden, severe, brief, stabbing, recurrent episodes of pain usually on one side of the face and can be provoked by light touch. The pain follows one or more branches of the trigeminal nerve which provides nerve sensation from the mouth, face and the front of the scalp.
The Phase II trial is a randomised, double-blind, placebo-controlled withdrawal study designed to evaluate the efficacy and safety of orally administered CNV1014802 in patients with TN. The trial will run in six countries and the first results are expected in mid 2013.
Commenting on the announcement Clive Dix, Chief Executive Officer of Convergence Pharmaceuticals, said: “We are delighted to announce the start of this Phase II trial in trigeminal neuralgia, our second clinical trial of CNV1014802, following a Phase II proof of concept study initiated in July 2011 for treating pain associated with lumbosacral radiculopathy (LSR). CNV1014802 has already demonstrated an excellent pharmacokinetic and safety profile in over 160 healthy volunteers in Phase I trials. TN is a very debilitating disorder and we are confident that CNV1014802 will help fill the desperate need for innovative new treatments. We look forward to reporting results in 2013.”
Most read news
Organizations
Other news from the department research and development

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Biotech Crops Poised for Second Wave of Growth - Political Will Strengthens Globally
Bradykinin
Nail_disease

Galapagos establishes strategic collaboration with BridGene Biosciences to expand small molecule drug discovery in oncology - Galapagos to pay up to $27 million in upfront and preclinical milestone payments to BridGene